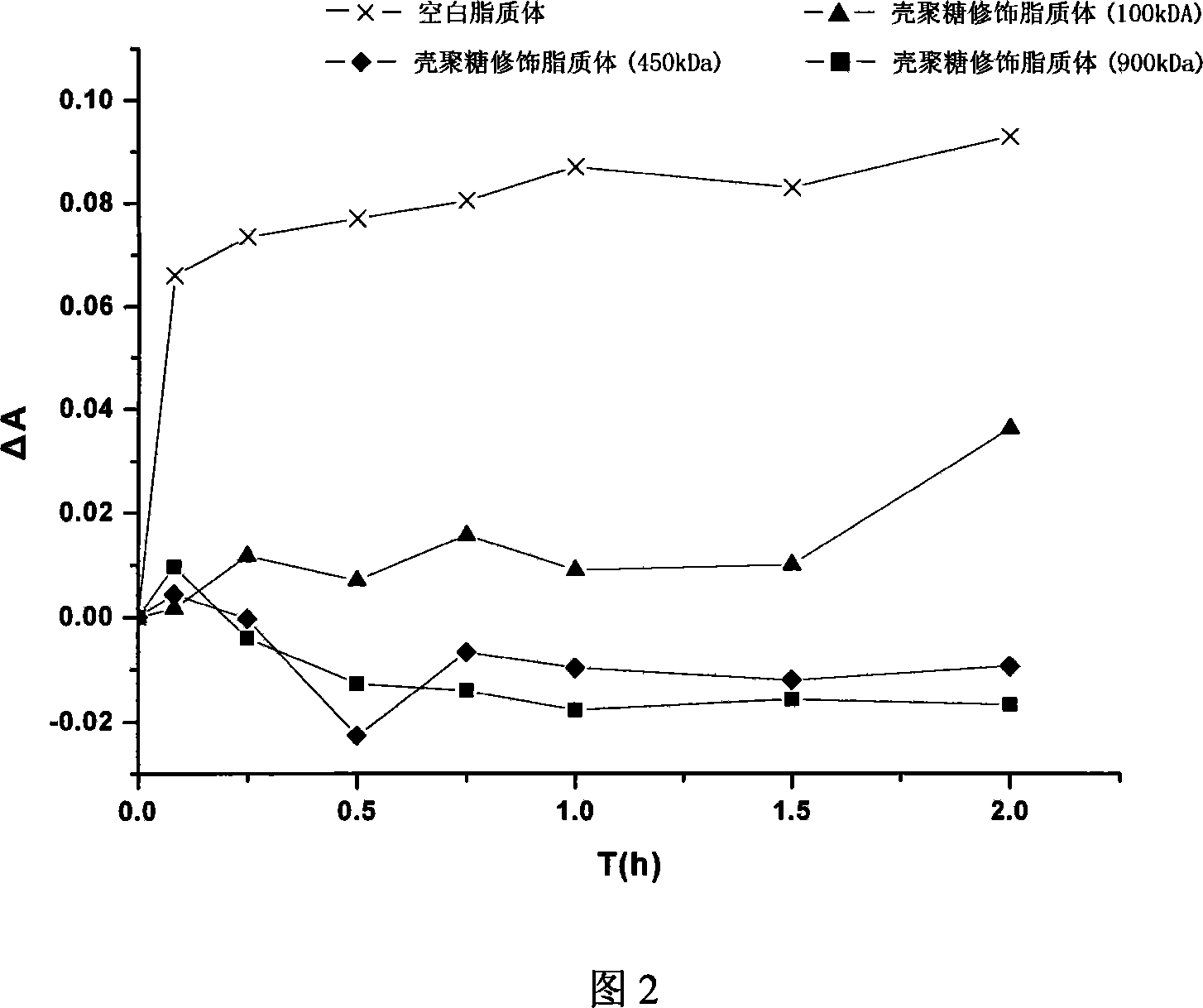
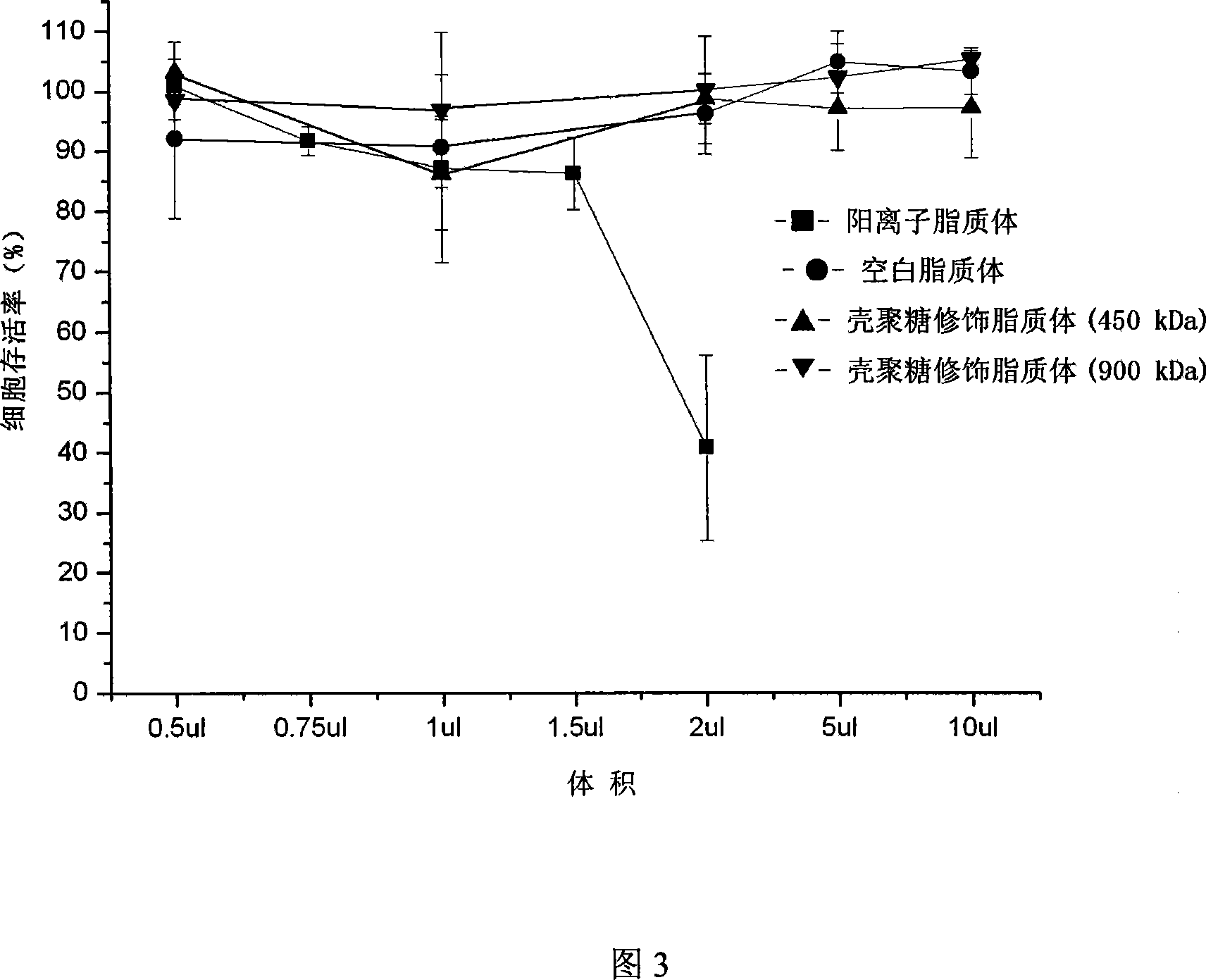
Method for preparing gene medication carrier by using chitosan modified liposome
A technology of drug delivery carrier and chitosan, applied in the field of gene drug delivery carrier preparation, can solve the problems of low transfection efficiency and low cytotoxicity, and achieve the effects of simple preparation process, good stability and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 Take 10 to 200 mg of soybean phospholipids and 5 to 100 mg of cholesterol to 7.5 to 150 mL of chloroform, add 2.5 to 50 mL of distilled water, and prepare liposomes by reverse evaporation. Specifically, it is vortexed for 10-30 minutes to make it into milk, and the chloroform is removed by vacuum rotary evaporation on a rotary evaporator to obtain a liposome suspension. The liposome solution is ultrasonically treated with a 100~500W probe, working time is 1~3 seconds, gap time is 2~4 seconds, cycle time is 30~240 times, ultrasonic condition is ice bath, squeezed through membrane, pore size is 0.1~0.22 μm. Take chitosan with a molecular weight of 450kDa and dissolve it in glacial acetic acid with a concentration of 0.1% to 1%. The final concentration of the chitosan solution is 0.2% to 1.0%. The liposome solution is slowly injected into the chitosan solution for 1-10 minutes, and then stirred for 0.5-2 hours to obtain chitosan modified liposomes.
Embodiment 2
[0014] Example 2 Take 10 to 200 mg of lecithin and 5 to 100 mg of cholesterol to 7.5 to 150 mL of chloroform, add 2.5 to 50 mL of distilled water, and prepare liposomes by reverse evaporation. The liposome solution is ultrasonically treated with a 100~500W probe, working time is 1~3 seconds, gap time is 2~4 seconds, cycle time is 30~240 times, ultrasonic condition is ice bath, squeezed through membrane, pore size is 0.1~0.22 μm. Take chitosan with a molecular weight of 900kDa and dissolve it in glacial acetic acid with a concentration of 0.1% to 1%. The final concentration of the chitosan solution is 0.2% to 1.0%. The liposome solution is slowly injected into the chitosan solution for 1-10 minutes, and then stirred for 0.5-2 hours to obtain chitosan modified liposomes.
[0015] Prepared by the thin film dispersion method. Specifically: taking 10 to 200 mg of soybean phospholipids and 5 to 100 mg of cholesterol in 7.5 to 150 mL of chloroform and placing them in a pear-shaped bottle...
Embodiment 4
[0016] Example 4 Take 10 to 200 mg of lecithin and 5 to 100 mg of cholesterol to 7.5 to 150 mL of chloroform, prepare according to the above-mentioned film dispersion method, and add 2.5 to 50 mL of distilled water for ultrasonic hydration. The liposome solution is ultrasonically treated with a 100~500W probe, working time is 1~3 seconds, gap time is 2~4 seconds, cycle time is 30~240 times, ultrasonic condition is ice bath, squeezed through membrane, pore size is 0.1~0.22 μm. Take chitosan with a molecular weight of 900kDa and dissolve it in glacial acetic acid with a concentration of 0.1% to 1%. The final concentration of the chitosan solution is 0.2% to 1.0%. The liposome solution is slowly injected into the chitosan solution for 1-10 minutes, and then stirred for 0.5-2 hours to obtain chitosan modified liposomes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com