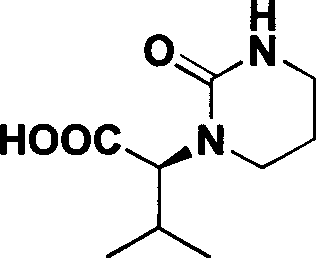
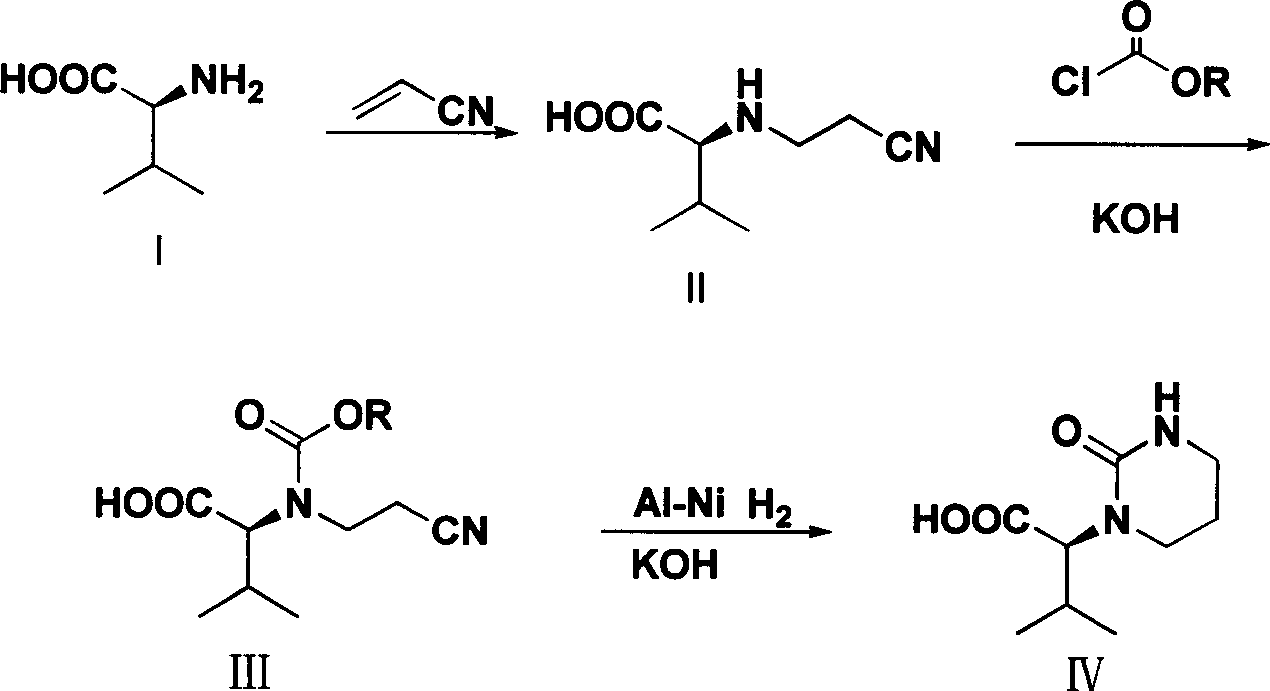
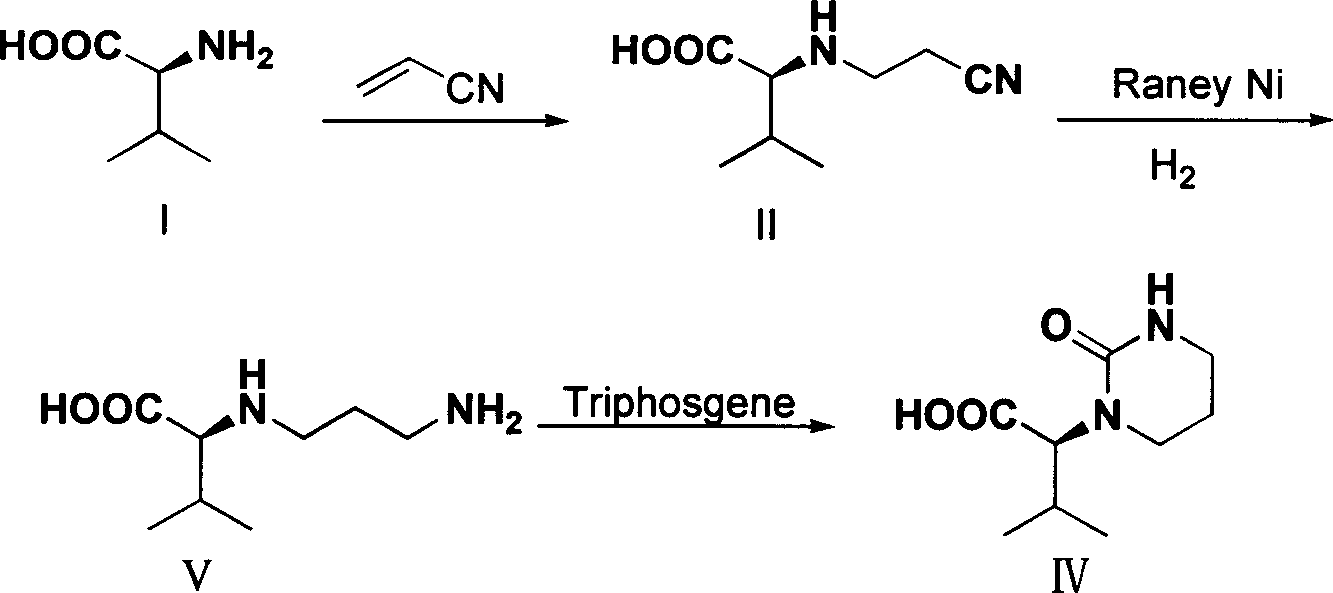
Process for preparing (S)-2-(2-carbonyl-tetrahydropyrimidyl-1-(2H)-base)-3-methyl butyric acid
A technology of methylbutyric acid and ectoine, which is applied in the field of preparation of -2--yl)-3-methylbutyric acid, can solve the problems of serious environmental pollution, low product yield, poor selectivity, etc., and achieve product High purity, high yield, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 (S)-2-(2-cyanoethylamino)-3-synthesis of methylbutanoic acid (compound II)
[0023] Add 11.7g of L-valine, 40ml of water, and 6.0g of NaOH into a 100mL three-necked flask. Slowly add 5.3 g of acrylonitrile dropwise to the system under the condition that the temperature of the system is controlled to be lower than 20° C., and the dropwise addition is completed within 30 minutes. After the dropwise addition, the system was controlled to react at 5-20°C for 20 hours. After the reaction is completed, adjust the pH of the system to 6-7 with concentrated hydrochloric acid, stir for 30 minutes, and then filter with suction. The resulting solid was washed with 40 mL of water. Drying gave 15.2 g of white solid compound II, yield: 90%.
[0024] 1 HNMR (300MHz, D 2 O): δ0.87(m, 6H, C(CH 3 ) 2 ), 1.78(m, 1H, CH), 2.57(m, 2H, CH 2 ), 2.69(m, 1H, N-CH), 2.81(m, 2H, CH 2 ).
Embodiment 2
[0025] The synthesis of embodiment 2 (S)-2-(2-cyanoethylamino)-3-methylbutyric acid (compound II)
[0026] Add 11.7g L-valine, 58ml water, 13.8gK 2 CO 3 . Slowly add 5.3 g of acrylonitrile dropwise to the system under the condition that the temperature of the system is controlled to be lower than 20° C., and the dropwise addition is completed within 30 minutes. After the dropwise addition, the system was controlled to react at 5-20°C for 10 hours. After the reaction is completed, adjust the pH of the system to 6-7 with concentrated hydrochloric acid, stir for 30 minutes, and then filter with suction. The resulting solid was washed with 40 mL of water. Drying gave 15.2 g of white solid compound II, yield: 90%.
[0027] 1 HNMR (300MHz, D 2 O): δ0.87(m, 6H, C(CH 3 ) 2 ), 1.78(m, 1H, CH), 2.57(m, 2H, CH 2 ), 2.69(m, 1H, N-CH), 2.81(m, 2H, CH 2 ).
Embodiment 3
[0028] The synthesis of embodiment 3 (S)-2-(2-cyanoethylamino)-3-methylbutanoic acid (compound II)
[0029] Add 11.7g of L-valine, 35ml of water, and 4.0g of NaOH into a 100mL three-necked flask. Slowly add 5.3 g of acrylonitrile dropwise to the system under the condition of controlling the system temperature at 50° C., and the dropwise addition is completed within 30 minutes. After the dropwise addition, the system was controlled to react at 50° C. for 5 hours. After the reaction is completed, adjust the pH of the system to 6-7 with concentrated hydrochloric acid, stir for 30 minutes, and then filter with suction. The resulting solid was washed with 40 mL of water. Drying gave 14.6g of white solid compound II, yield: 86%.
[0030] 1 HNMR (300MHz, D 2 O): δ0.87(m, 6H, C(CH 3 ) 2 ), 1.78(m, 1H, CH), 2.57(m, 2H, CH 2 ), 2.69(m, 1H, N-CH), 2.81(m, 2H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com