Gold plating solution, and method for plating gold
A technology of gold plating solution and gold salt, applied in the field of gold plating solution and its gold plating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
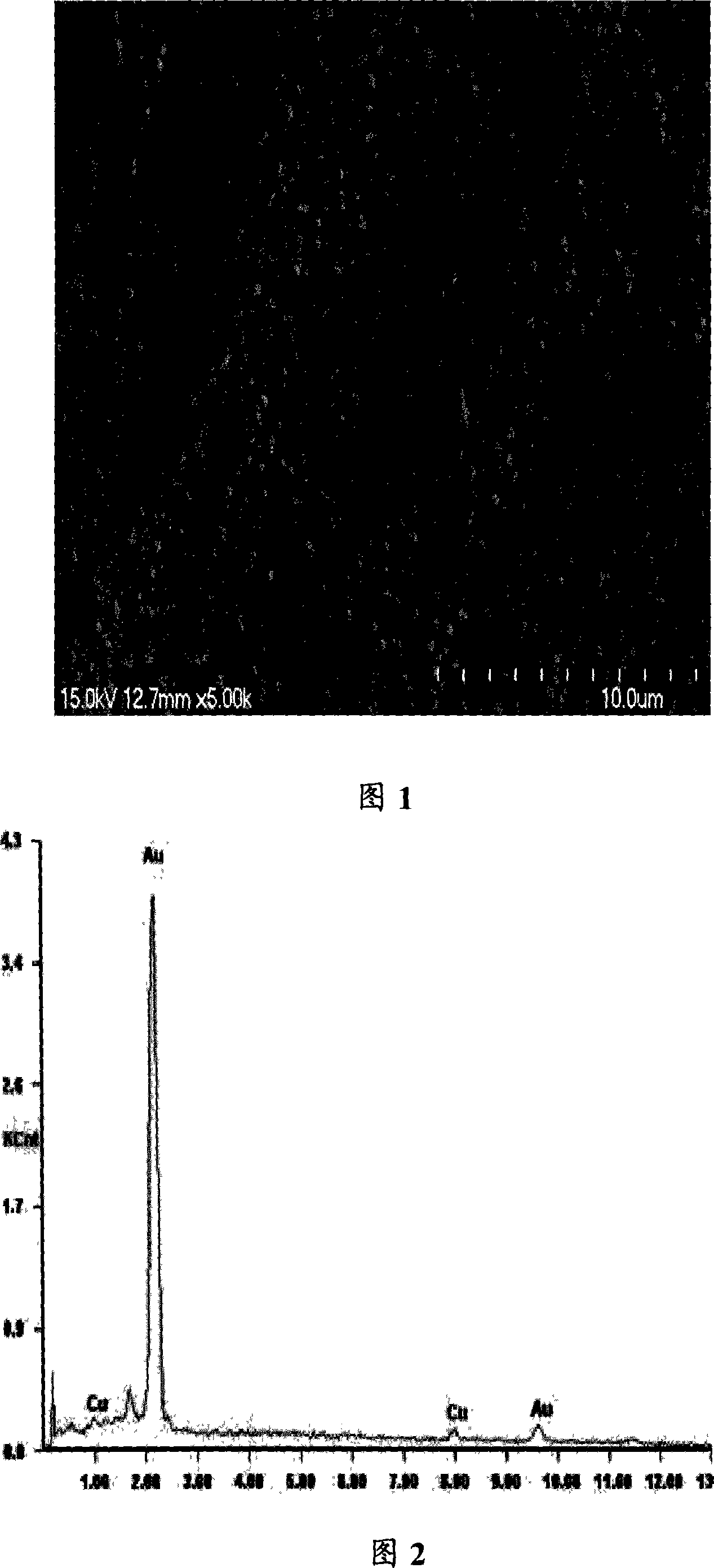
[0025] Example 1: In a 25ml single-chamber electrolyzer, add 0.05 grams of Au(PPh 3 )Cl and 10ml ionic liquid [bmim]BF 4 , The temperature was raised to 80°C to fully dissolve the gold salt in the ionic liquid. Using the gold salt-containing ionic liquid as electrolyte, direct current electroplating, using gold sheet (10mm×12mm, purity of 99.9%) as anode, copper sheet (12mm×14mm, purity of 99.5%) as cathode, and the cathode and anode are Aligned vertically, the electrode surfaces are parallel to each other, stirring, current density 0.08A / dm 2 , The cell voltage is 1.8~2.0V, the pole pitch is 10mm, and the plating temperature is 80℃. A bright gold coating was obtained after 2 hours of electrification. See Figure 1 and Figure 2 for the effect.
Embodiment 2
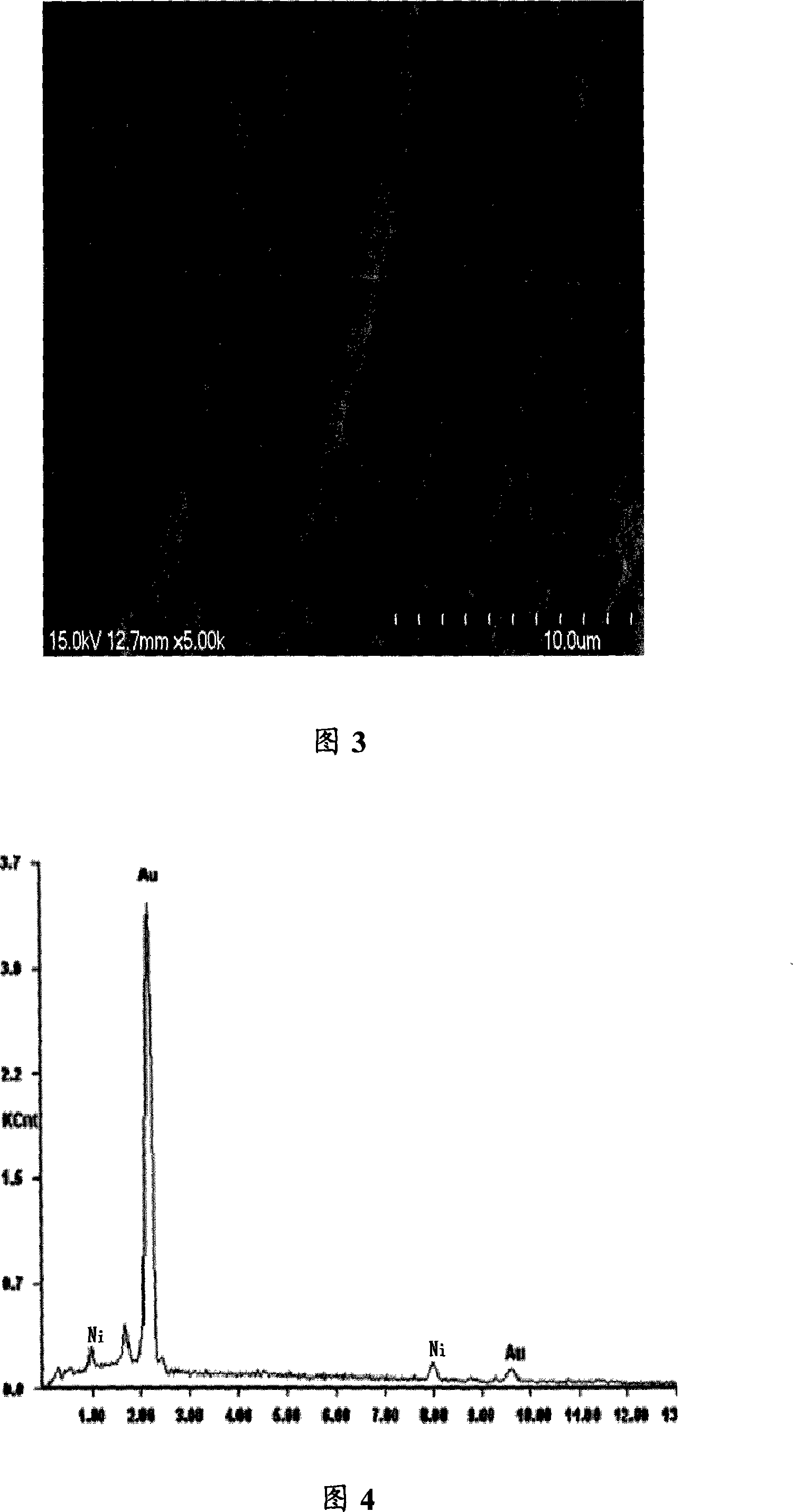
[0026] Example 2: In a 25ml single-chamber electrolyzer, add 0.05 grams of Au(PPh 3 )Cl and 10ml ionic liquid [bmim]BF 4 , The temperature was raised to 90°C to fully dissolve the gold salt in the ionic liquid. Using the gold salt-containing ionic liquid as electrolyte, direct current electroplating, using gold sheet (10mm×12mm, purity of 99.9%) as anode, nickel sheet (8mm×10mm, purity of 99.5%) as cathode, the cathode and anode are Aligned vertically, the electrode surfaces are parallel to each other, stirring, current density 0.18A / dm 2 , The cell voltage is 1.8~2.0V, the pole distance is 15mm, and the plating temperature is 90℃. A bright gold coating was obtained after 2 hours of electrification.
Embodiment 3
[0027] Example 3: In a 25ml single-chamber electrolyzer, add 0.05 grams of Au(PPh 3 )Cl and 10ml ionic liquid [bmim]PF 6 , The temperature was raised to 90°C to fully dissolve the gold salt in the ionic liquid. Using the gold salt-containing ionic liquid as electrolyte, direct current electroplating, using gold sheet (10mm×12mm, purity of 99.9%) as anode, copper sheet (12mm×14mm, purity of 99.5%) as cathode, and the cathode and anode are Aligned vertically, the electrode surfaces are parallel to each other, stirring, current density 0.05A / dm 2 , Cell voltage 2.0~2.3V, pole distance 10mm, electrolysis temperature 90℃. A bright gold coating was obtained after 2 hours of electrification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com