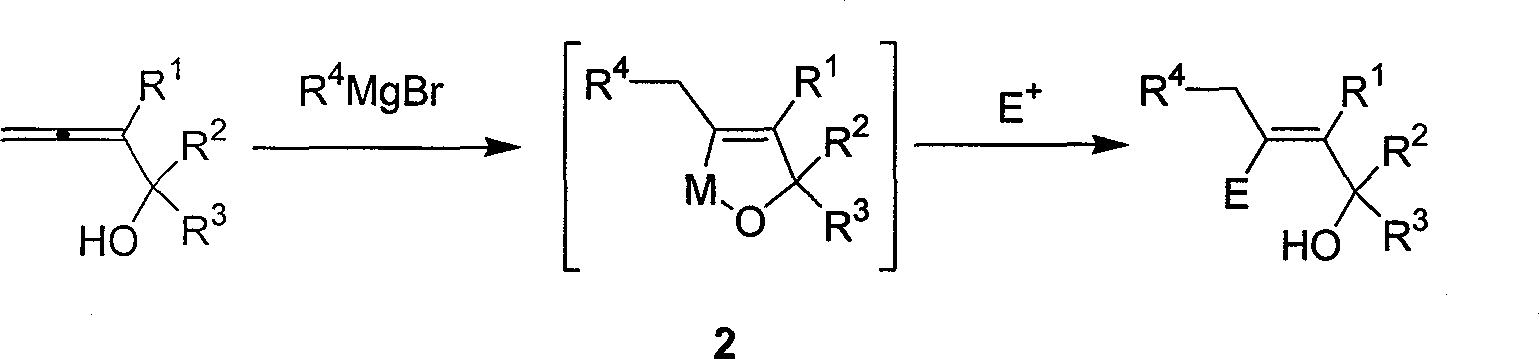
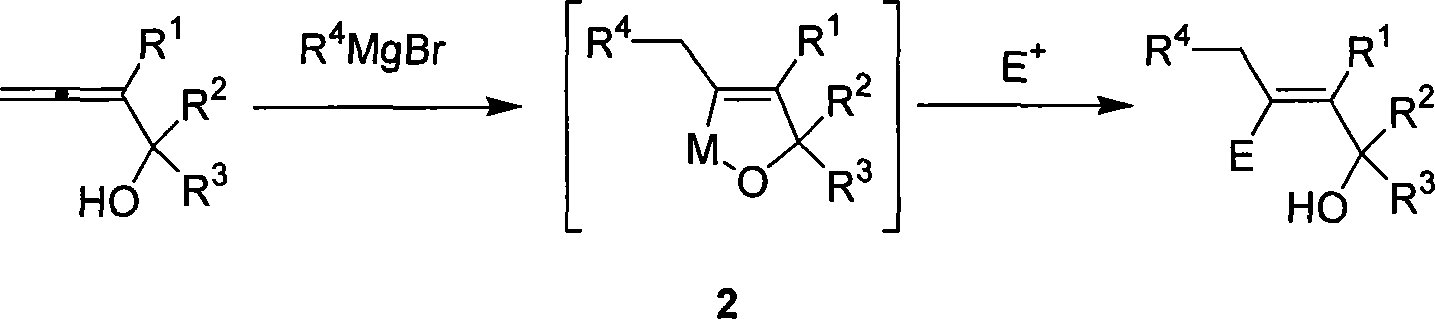
Method for synthesizing beta-functionalized multi-substituted allyl alcohol
A technology of functional grouping and allyl alcohol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as low selectivity, difficulty in repeating, inapplicability of secondary Grignard reagents, etc., and achieve easy The effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen, add cuprous chloride (0.1974g, 2.0mmol), 2-butyl-2,3-butadien-1-ol (0.1244g, 1.0mmol) and ether (1.5mL), cool to minus seven Eighteen degrees, dropwise added to the ether solution (5mL, 5mmol) containing amyl Grignard reagent, after the addition, slowly return to room temperature, react overnight, then cool to minus seventy-eight degrees, dropwise add iodine (1.0g , 4 mmol) in tetrahydrofuran solution, the reaction was completed under stirring for 0.5 hours, and saturated sodium thiosulfate was added dropwise to eliminate excess iodine. Extracted with ether, washed with saturated sodium thiosulfate, 5% hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and flash column chromatography gave 0.2254 g of the product with a yield of 70 %. The product is a colorless liquid.
[0026] 1 H NMR (400MHz, CDCl 3 )δ4.22(s, 2H), 2.54(t, J=7.6Hz, 2H), 2.30(t, J=8.0Hz, 2H), 1.68(bs, 1H),...
Embodiment 2
[0031] Under nitrogen, add cuprous chloride (0.1975g, 2.0mmol), 3-butyl-3,4-pentadien-2-ol (0.1401g, 1.0mmol) and ether (1.5mL), cool to minus seven Eighteen degrees, dropwise added to the ether solution containing amyl Grignard reagent (5mL, 5mmol), after the addition, slowly return to room temperature, react overnight, then cool to minus five degrees, dropwise add iodine (1.27g, 5mmol ) THF solution, the reaction was complete under stirring for 0.5 hour, and saturated sodium thiosulfate was added dropwise to eliminate excess iodine. Extracted with ether, washed with saturated sodium thiosulfate, 5% hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and flash column chromatography gave the product 0.1908g with a yield of 56 %. The product is a colorless liquid.
[0032] 1 H NMR (400MHz, CDCl 3 )δ4.77(q, J=6.3Hz, 1H), 2.55-2.43(m, 2H), 2.30-2.20(m, 1H), 2.20-2.10(m, 1H), 1.67(bs, 1H), 1.58 -1....
Embodiment 3
[0034] According to the method described in Example 2, the difference is that the substrate used and the reagent are: cuprous chloride (0.1998g, 2.0mmol), S-3-butyl-3,4-pentadien-2-alcohol ( 0.1401g, 1.0mmol, ee=98%) the diethyl ether solution (5mL, 5mmol) of pentyl Grignard reagent, iodine (1.3017g, 5mmol), obtains the S configuration product 0.2745g, and yield rate is 81% (ee=98%) %). The product is a colorless liquid.
[0035] 1 H NMR (400MHz, CDCl 3)δ4.77(q, J=6.3Hz, 1H), 2.55-2.43(m, 2H), 2.30-2.20(m, 1H), 2.20-2.10(m, 1H), 1.67(bs, 1H), 1.58 -1.50(m, 2H), 1.50-1.26(m.10H), 1.23(d, J=6.3Hz, 3H), 0.95-0.85(m, 6H); 13 C NMR (CDCl 3 , 100MHz) δ145.4, 105.6, 77.7, 41.1, 33.0, 31.7, 29.7, 28.2, 27.6, 23.1, 22.6, 20.8, 14.0, 13.8; MS (m / z) 338 (M + , 1.69), 211 (M + -I, 23.07), 43 (100); IR (neat, cm -1 )3373, 2957, 2926, 2858, 1618, 1465, 1378, 1111, 1060; HRMS calcd for C 14 h 27 OI 338.1107, found 338.1115.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com