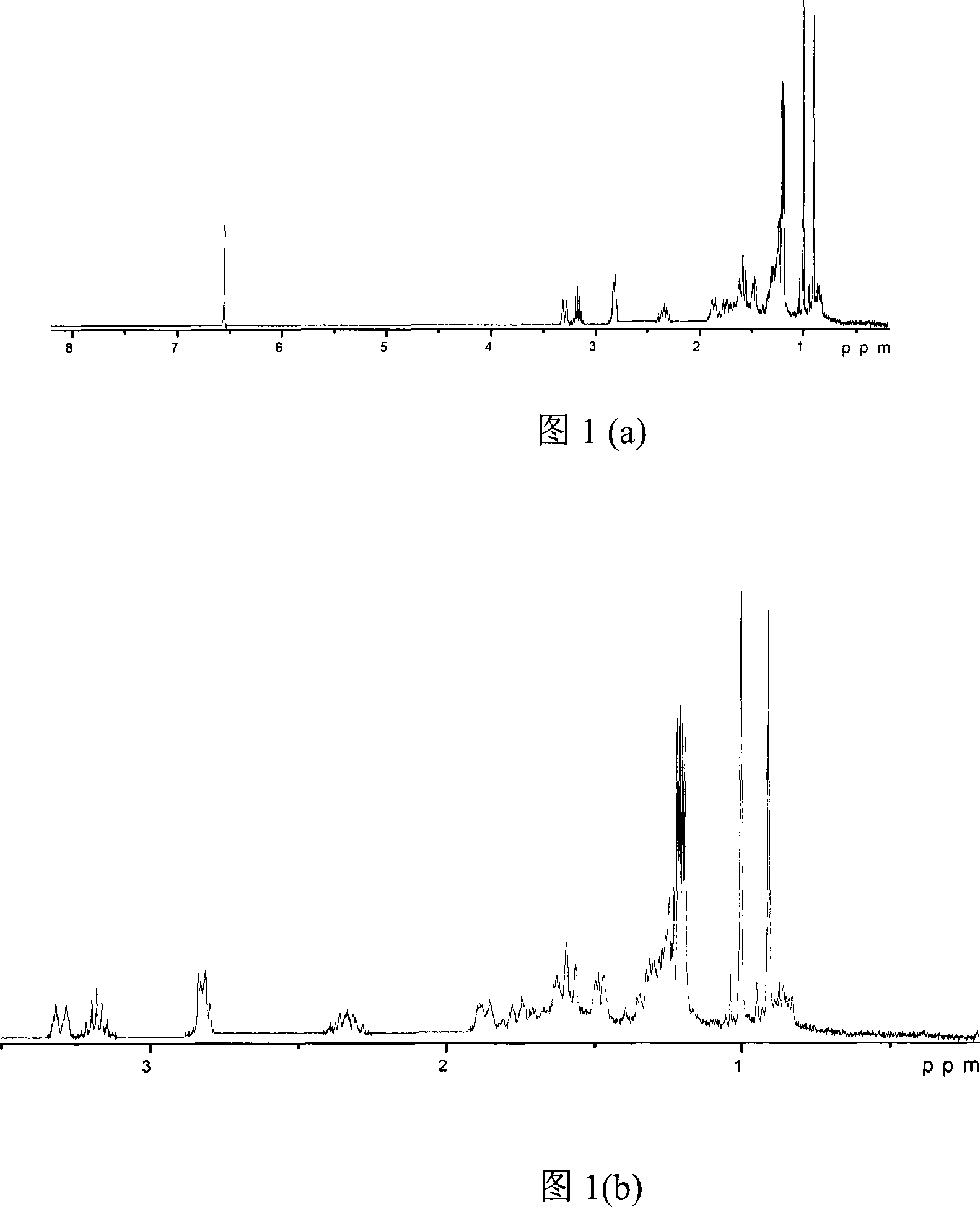
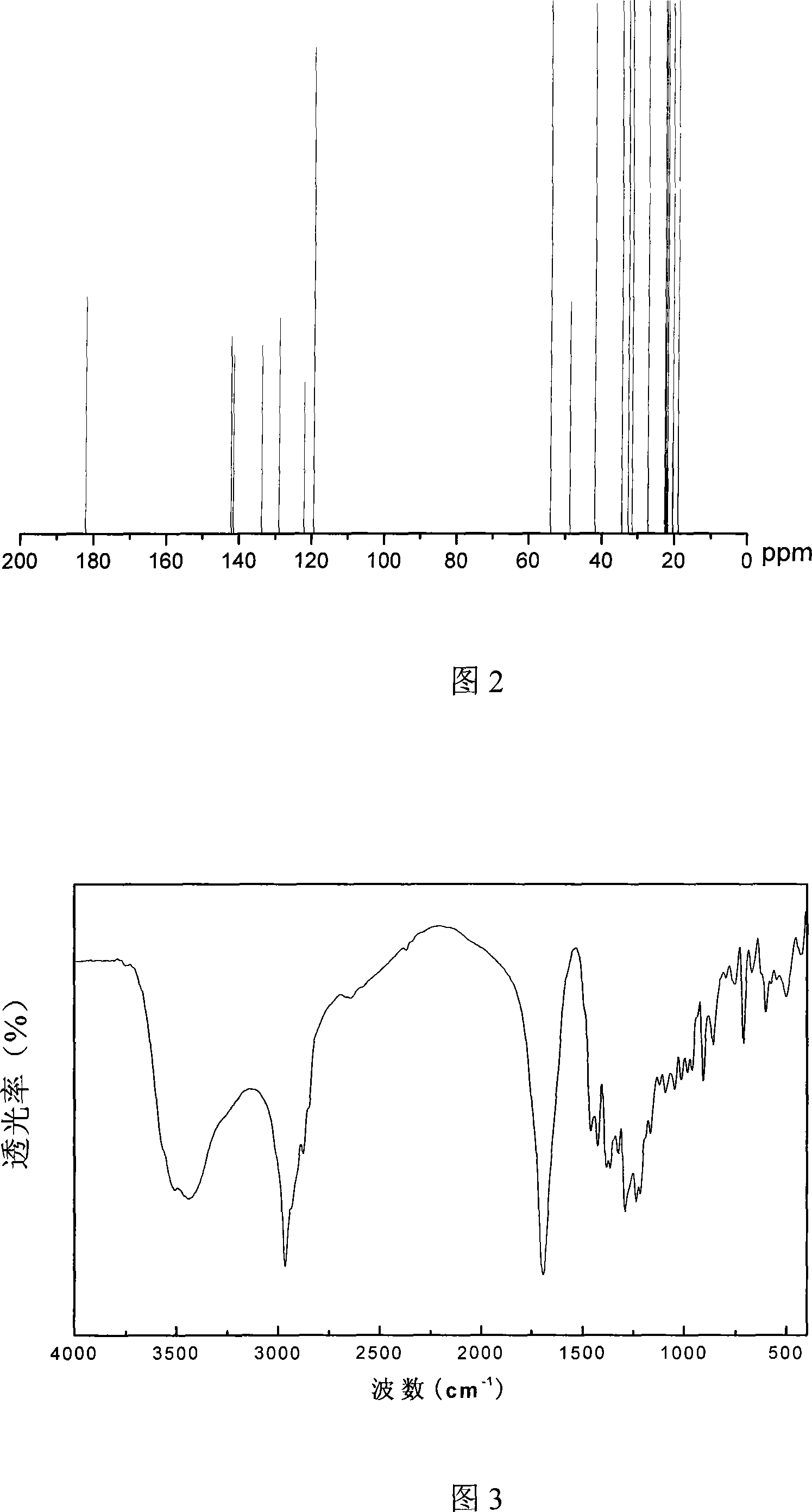
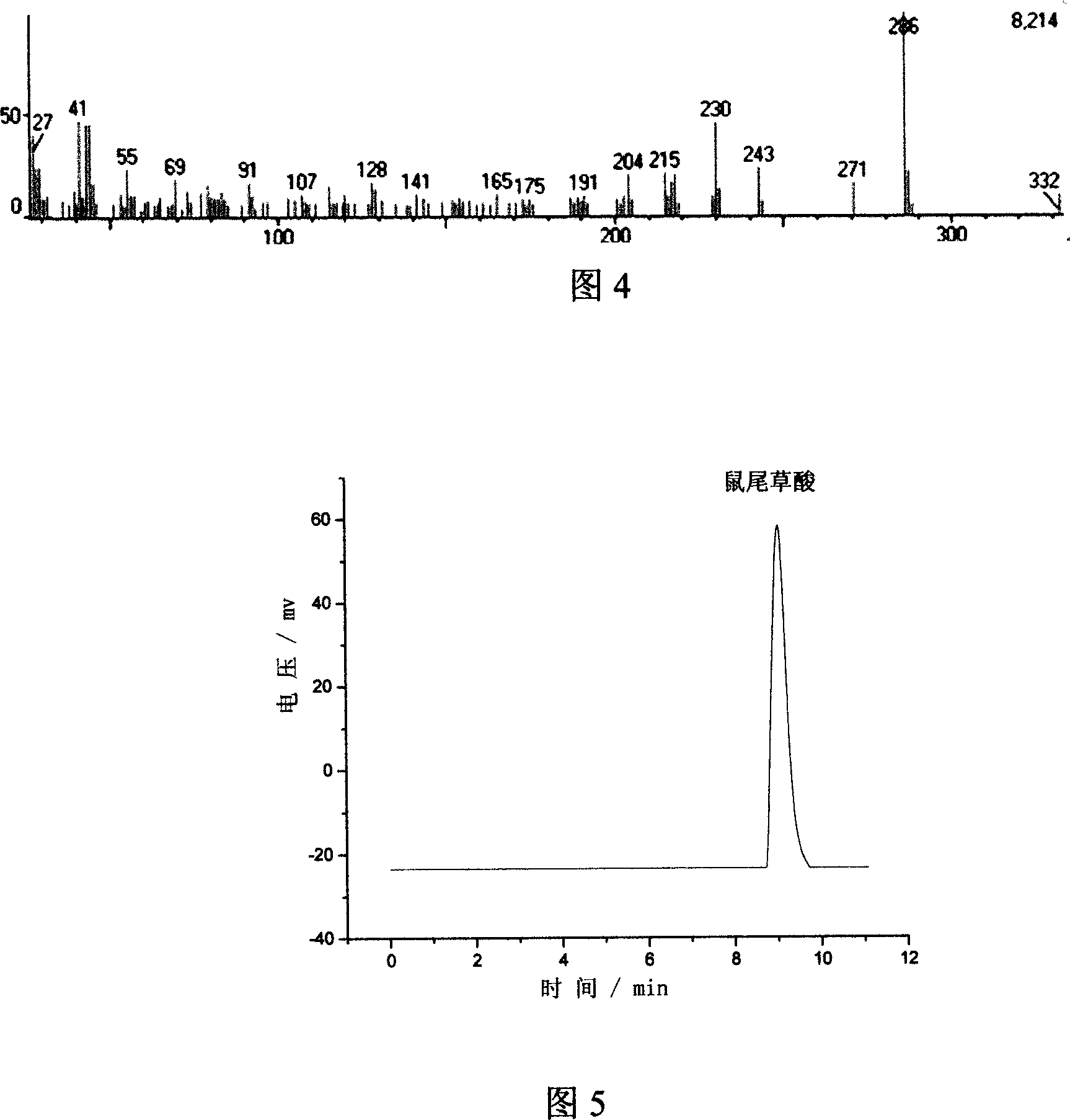
Method for extracting carnosic acid
A technology of carnosic acid and extraction method, which is applied in the separation/purification of carboxylic acid compounds, organic chemistry and other directions, can solve the problems of many by-products, complex reactions, and difficulty in industrialization, and achieves the effect of short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 30 grams of rosemary dry leaves (commercially available, the same below), soak in 180ml of acetone (the mass of rosemary dry leaves: the volume of organic solvent is 1:6), ultrasonically extract for 40min at 35°C, filter to obtain grass green rosemary Fragrant Dried Leaf Extract. Add 9 grams of activated carbon (the mass ratio of activated carbon: rosemary dry leaf extract is 1:20) to the grass-green rosemary dry leaf extract, and ultrasonically decolorize it at 40° C. for 10 min. Filter twice to obtain a brown-red transparent extract. The brown-red transparent extract was evaporated to dryness of acetone with a rotary evaporator to obtain a yellow-brown solid. Add EDTA aqueous solution (concentration of EDTA aqueous solution is 90g / L; yellowish brown solid mass:volume of EDTA aqueous solution=1:20) to tan solid to disperse the solid, add anhydrous Na2 CO 3 (90g / L) to adjust the pH value to 8.5 (NaCO 3 The amount of the substance is about 2.5 times that of the ...
Embodiment 2
[0039] Weigh 30 grams of rosemary dry leaves, soak them in 180ml of acetone (the mass of rosemary dry leaves: the volume of acetone is 1:6), ultrasonically extract at 40° C. for 30 min, and filter to obtain the grass-green rosemary dry leaf extract. Add 9 grams of activated carbon (the mass ratio of activated carbon:dried rosemary leaf extract is 1:20) to the grass-green rosemary dry leaf extract, and ultrasonically decolorize at 45° C. for 5 min. Filter twice to obtain a brown-red transparent extract. The brown-red transparent extract was evaporated to dryness of acetone with a rotary evaporator to obtain a yellow-brown solid. Add EDTA aqueous solution (concentration of EDTA aqueous solution is 90g / L; yellowish brown solid mass:volume of EDTA aqueous solution=1:20) to tan solid to disperse the solid, add anhydrous Na 2 CO 3 (90g / L) to adjust the pH value to 8.3 (NaCO 3 The amount of the substance is about 2.5 times that of the carnosic acid, and the mass content of the car...
Embodiment 3
[0041] Take by weighing 30 grams of rosemary dry leaves, soak in 180ml ethanol (the ratio of rosemary dry leaves mass: ethanol volume is 1: 6), ultrasonically extract at 30°C for 45min, filter to obtain grass green rosemary dry leaf extract. Add 9 grams of activated carbon (the mass ratio of activated carbon: rosemary dry leaf extract is 1:20) to the grass-green rosemary dry leaf extract, and ultrasonically decolorize at 30° C. for 15 min. Filter twice to obtain a brown-red transparent extract. The brown-red transparent extract was evaporated to dry ethanol with a rotary evaporator to obtain a yellow-brown solid. Add EDTA aqueous solution (concentration of EDTA aqueous solution is 90g / L; yellowish brown solid mass:volume of EDTA aqueous solution=1:20) to tan solid to disperse the solid, add anhydrous Na 2 CO 3 (90g / L) to adjust the pH value to 8.5 (NaCO 3 The amount of the substance is about 2.5 times that of the carnosic acid, and the mass content of the carnosic acid is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com