Method for synthesizing bicycle aza ring fluorescent or phosphorescent compound
A synthesis method and compound technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as low efficiency, difficult synthesis, and many synthesis steps, and achieve mild reaction conditions, fewer reaction steps, and excellent yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
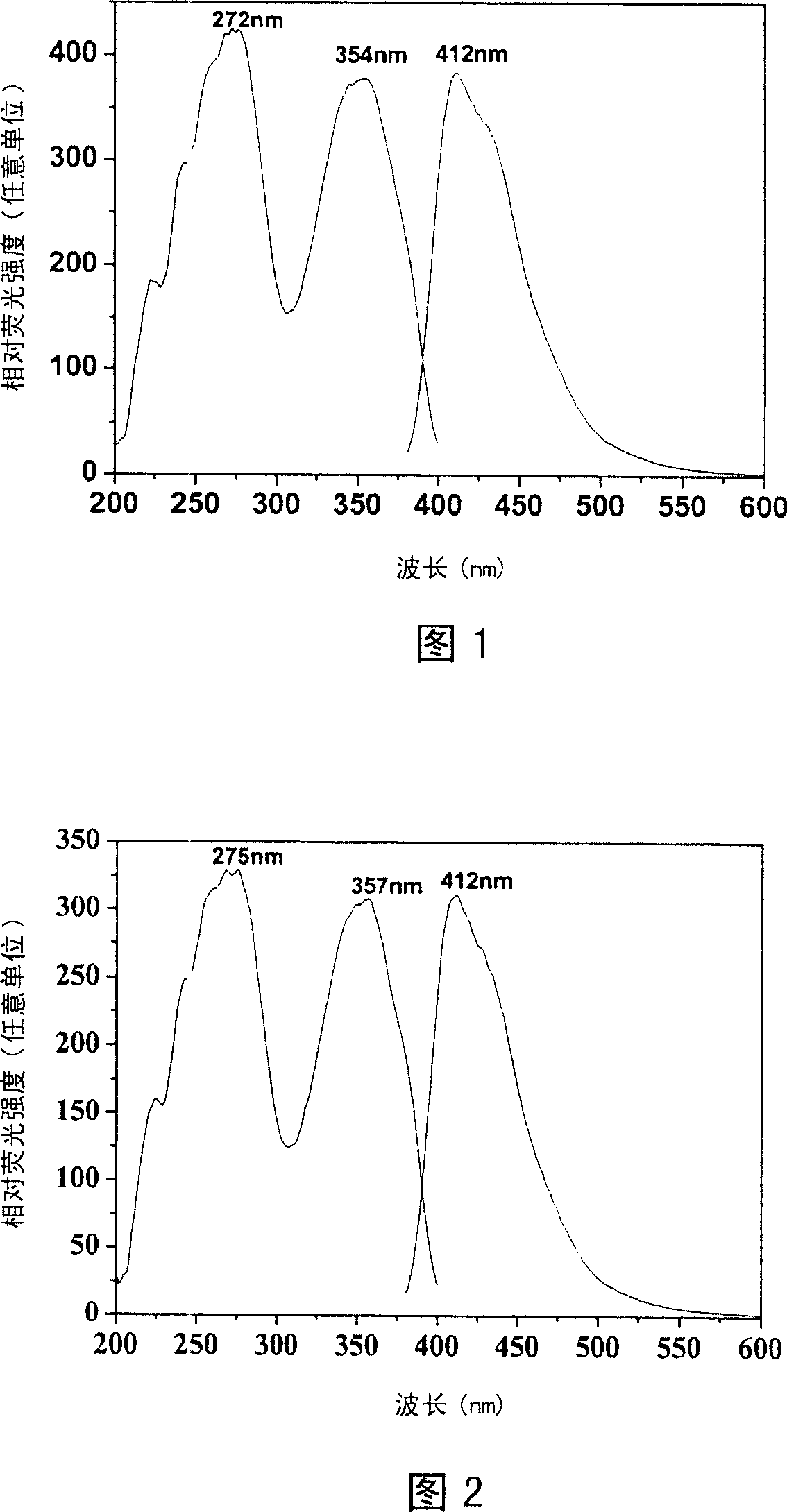
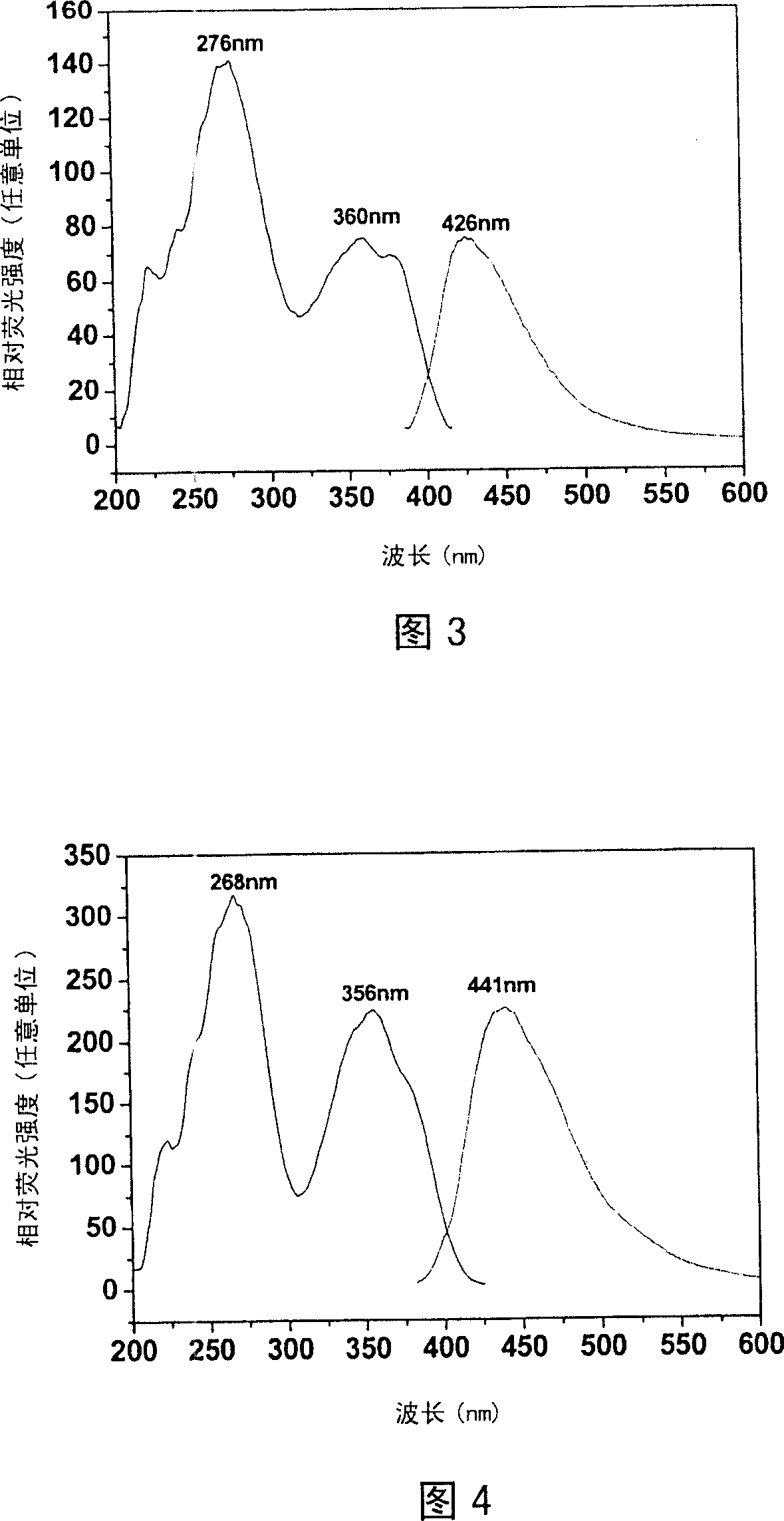
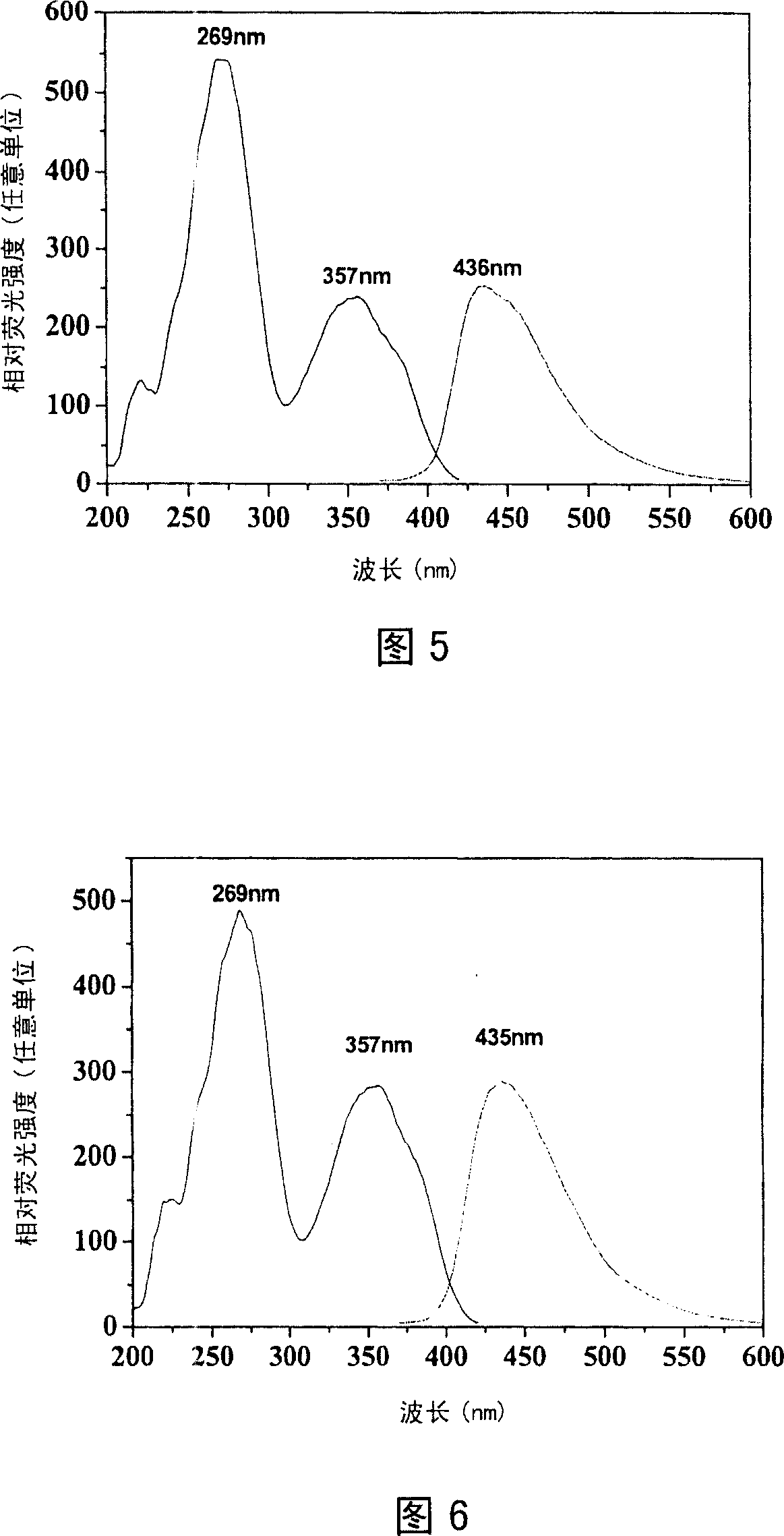
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of bicyclic nitrogen heterocyclic carbene compound 3a and 4a
[0050] Put Fischer carbene compound 1a (1.0 mmol), 3-methyl-2-pyrazolin-5-one 2a (1.0 mmol) into a 5 ml reaction flask, and add 3 ml of tetrahydrofuran (THF). The whole reaction device was stirred in a 50° C. oil bath for 30 minutes. After the reaction was completed, the reaction mixture could be separated and purified by column chromatography. The chromatographic column was a silica gel column. When the eluent was petroleum ether (30~60° C.) / dichloromethane ( The yield of purple fraction 3a was 35.1% when v / v, 4:1); the blue fraction was collected when the eluent was petroleum ether (30-60°C) / dichloromethane (v / v, 2:1) Fraction 4a yield was 23.4%.
Embodiment 2
[0051] Embodiment 2: the synthesis of bicyclic nitrogen heterocyclic carbene compound 3b and 4b
[0052] Put Fischer carbene compound 1b (1.0 mmol), 3-methyl-2-pyrazolin-5-one 2a (1.0 mmol) into a 5 ml reaction flask, and add 3 ml of tetrahydrofuran (THF). The whole reaction device was stirred in a 50° C. oil bath for 30 minutes. After the reaction was completed, the reaction mixture could be separated and purified by column chromatography. The chromatographic column was a silica gel column. When the eluent was petroleum ether (30~60° C.) / dichloromethane ( The yield of purple fraction 3b was 46.4% when v / v, 4:1); the blue fraction was collected when the eluent was petroleum ether (30~60°C) / dichloromethane (v / v, 2:1) The yield of fraction 4b was 23.0%,
Embodiment 3
[0053] Embodiment 3: the synthesis of bicyclic nitrogen heterocyclic carbene compound 3c and 4c
[0054] Put Fischer carbene compound 1a (1.0 mmol), 3-propyl-2-pyrazolin-5-one 2b (1.0 mmol) into a 5 ml reaction flask, and add 3 ml of tetrahydrofuran (THF). The whole reaction device was stirred in a 50° C. oil bath for 30 minutes. After the reaction was completed, the reaction mixture could be separated and purified by column chromatography. The chromatographic column was a silica gel column. When the eluent was petroleum ether (30~60° C.) / dichloromethane ( The yield of purple fraction 3c was 46.7% when v / v, 4:1); the blue fraction was collected when the eluent was petroleum ether (30-60°C) / dichloromethane (v / v, 2:1) The yield of fraction 4c was 16.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com