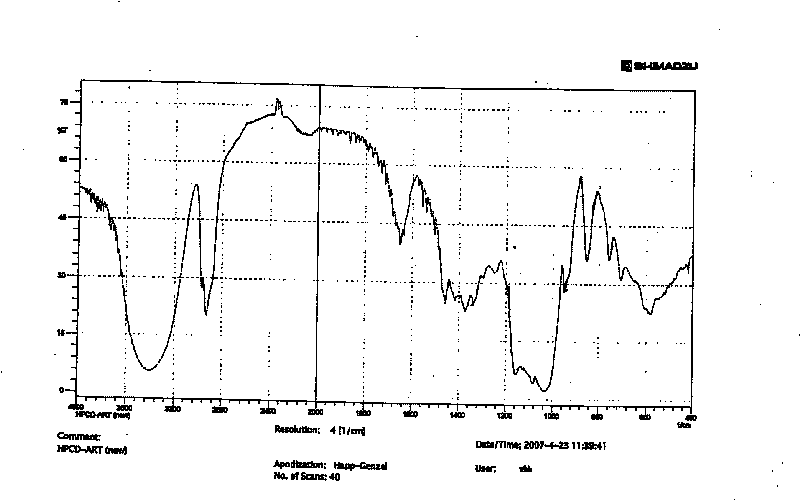
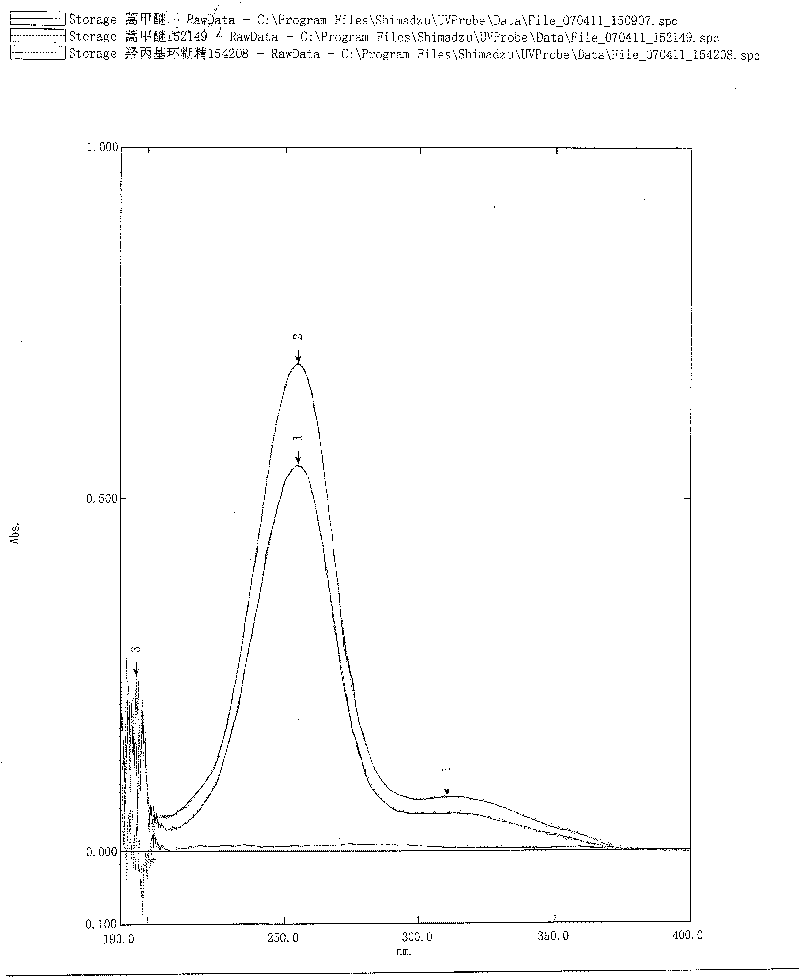
Artemisinin derivatives freeze-dried preparation and preparation method
A technology for freeze-dried preparations and derivatives, which is applied in the field of freeze-dried preparations and preparations of artemisinin derivatives, which can solve problems such as organic solvent residues and microbial contamination, and achieve the effects of short production cycle, fast dissolution speed, and investment saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] formula:
[0029] Artemether (conventional refined powder, particle size greater than 315μm) 60g
[0030] Hydroxypropyl-β-cyclodextrin 2350g
[0031] Made into 1000
[0032] Weigh the prescription amount of hydroxypropyl-β-cyclodextrin, first dissolve 1600g in 4000ml water for injection to make a concentration of 40%, heat it to 70℃~90℃, add the prescription amount of artemether (particle size greater than 315μm), stir, add the remaining hydroxypropyl-β-cyclodextrin, stir to dissolve, add water for injection to 5000ml, fill and freeze-dry to obtain the finished product.
Embodiment 2
[0034] formula:
[0035] Artemether (fine powder 1.0μm~10μm) 60g
[0036] Hydroxypropyl-β-cyclodextrin 2200g
[0037] Made into 1000
[0038] A low-temperature pulverizer (0°C-10°C) is used to pulverize artemether to obtain artemether with a particle size of 1.0μm-10μm. Separately weigh the prescription amount of hydroxypropyl-β-cyclodextrin, first dissolve 1800g in 4000ml water for injection to make 45% concentration, heat to 50℃~60℃, add prescription amount of artemether, stir, Then add the remaining hydroxypropyl-β-cyclodextrin, stir to dissolve, add water for injection to 5000ml, fill and freeze-dry to obtain the finished product.
Embodiment 3
[0040] formula:
[0041] Artemether (micropowder 1.0μm~20μm) 80g
[0042] Sulfobutyl ether-β-cyclodextrin 440
[0043] Methyl-β-cyclodextrin 2000
[0044] Made into 1000
[0045] A low-temperature pulverizer (0°C-10°C) is used to pulverize artemether to obtain artemether with a particle size of 1.0μm-20μm. Separately weigh the prescribed amount of sulfobutyl ether-β-cyclodextrin and methyl-β-cyclodextrin, first dissolve 1200g of methyl-β-cyclodextrin in 4000ml water to make a concentration of 30%, add Warm to 50℃~60℃, add the prescription amount of artemether, stir, then add the remaining methyl-β-cyclodextrin and sulfobutyl ether-β-cyclodextrin, stir to dissolve, add water for injection until 5000ml, filled and freeze-dried to get the finished product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com