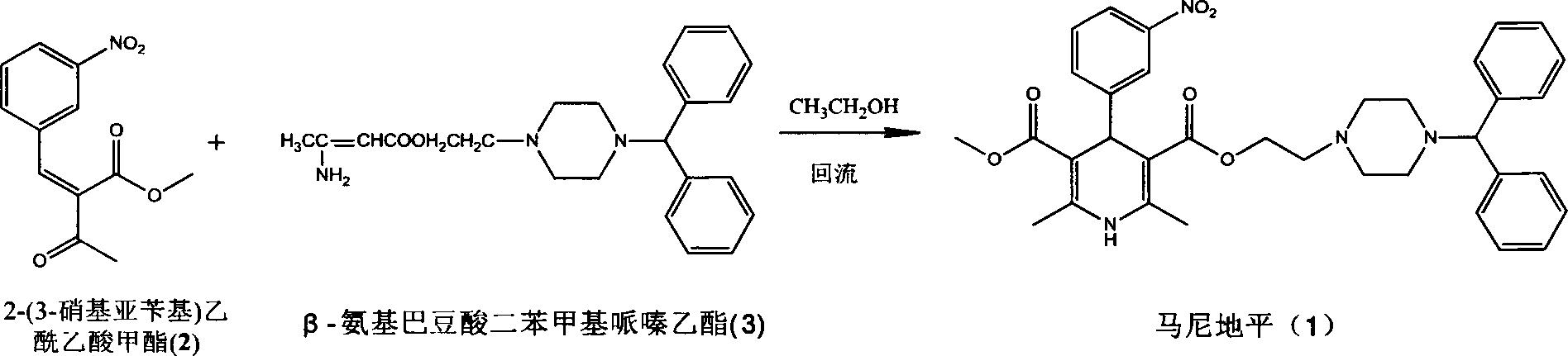
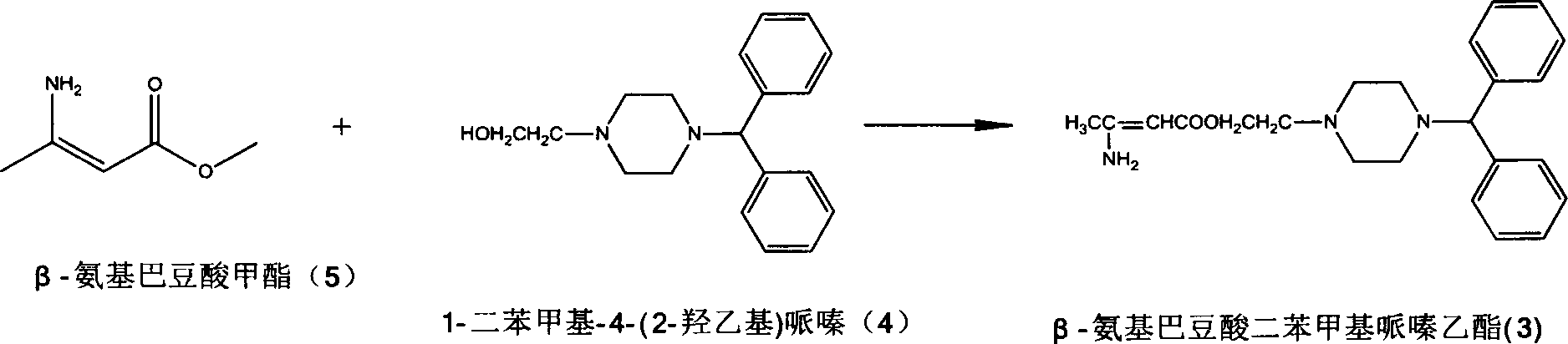
Calcium ion channels antagonist manidipine preparation method
A technology of calcium ion channel and manidipine, which is applied in the field of drug preparation, can solve the problems of complex by-products of reaction products, difficulty in obtaining intermediates, unsuitable for industrial production, etc., and achieve less impurities in the reaction process, simple post-treatment, and technological conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of benzhydrylpiperazine ethyl β-aminocrotonate (3)
[0019] Dissolve methyl β-aminocrotonate (20g, 0.174mol) in chloroform (100ml), add sodium methoxide (11.28g, 0.209mol), and add 1-benzhydryl-4-(2- Hydroxyethyl)piperazine (77.17g, 0.260mmol) in chloroform (50ml) was heated to reflux for 6h. Water (50ml×3) was added for extraction, the organic phase was evaporated under reduced pressure to remove the organic solvent, and petroleum ether was added for crystallization to obtain 55.10 g of solid, with a yield of 83.58%.
Embodiment 2
[0021] Preparation of benzhydrylpiperazine ethyl β-aminocrotonate (3)
[0022] Methyl β-aminocrotonate (20g, 0.174mol) was dissolved in toluene (100ml), potassium carbonate (24.05g, 0.174mol) was added, and 1-benzhydryl-4-(2- Hydroxyethyl) piperazine (56.68g, 0.191mmol) in toluene (50ml) solution, keep warm for 4h. The solvent was evaporated under reduced pressure, extracted with water (300ml) and dichloromethane (80ml×3), the organic phases were combined, dried, and the organic solvent was evaporated to remove the organic solvent. The resulting product was crystallized with petroleum ether to obtain 52.66g of solid, with a yield of 79.88%.
Embodiment 3
[0024] Preparation of benzhydrylpiperazine ethyl β-aminocrotonate (3)
[0025] Methyl β-aminocrotonate (20g, 0.174mol) was dissolved in toluene (100ml), sodium methoxide (11.28g, 0.209mol) was added, and 1-benzhydryl-4-(2- Hydroxyethyl) piperazine (51.53g, 0.174mmol) in toluene (50ml) solution, keep warm for 4h. The solvent was evaporated under reduced pressure, extracted with water (150ml) and dichloromethane (50ml×3), the organic phases were combined, dried, and the organic solvent was evaporated to remove the organic solvent. The resulting product was crystallized with petroleum ether to obtain 53.89g of solid, with a yield of 81.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com