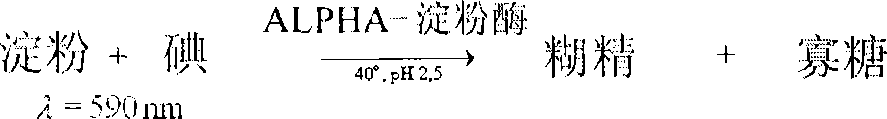Enzymes for starch processing
A technology of amylase and amino acid, applied in the field of peptides, can solve problems such as energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0094] In a preferred embodiment, the polypeptide comprises a CBM derived from Atathelia rotundum, A. papyrus, Valsaria rubricosa, or A. macroporus. Preferably any polypeptide comprising a CBM amino acid sequence selected from the group consisting of: A. papierae glucoamylase (SEQ ID NO: 92), A. papieriformis glucoamylase (SEQ ID NO: 76), Valsaria rubricosa α - Amylase (SEQ ID NO: 84) and Macroporus alpha-amylase (SEQ ID NO: 88).
[0095] In another preferred embodiment, the polypeptide comprises an α-amylase sequence (SEQ ID NO: 4) derived from Aspergillus oryzae acid α-amylase, preferably wherein the Aspergillus oryzae amino acid sequence comprises one selected from the following group or multiple amino acid substitutions: A128P, K138V, S141N, Q143A, D144S, Y155W, E156D, D157N, N244E, M246L, G446D, D448S, and N450D. Most preferably said polypeptide comprises a catalytic domain having the amino acid sequence shown in SEQ ID NO:6. In a preferred embodiment, the polypeptide f...
Embodiment 1
[0285] Embodiment 1: Construction of the nucleic acid sequence V019 encoding Rhizomucor pusillus (Rhizomucor pusillus) α-amylase and Athelia rolfsii (Athelia rolfsii) glucoamylase CBM
[0286] Vector pLA1 was digested with appropriate restriction endonucleases to excise the region encoding the catalytic domain of the A. niger alpha-amylase. Primers P001 (SEQ ID NO: 104) and P002 (SEQ ID NO: 105) were used to amplify the Rhizomucor pumila α-amylase gene by PCR, and the amplified fragment is shown in SEQ ID NO: 19.
[0287] PCR reaction system:
[0288] DNA fragments were recovered from agarose gels using a Qiagen gel extraction kit. The resulting purified fragments were mixed with the vector digest. The mixed solution was introduced into Saccharomyces cerevisiae to construct expression plasmid pLAV019 by in vivo recombination.
Embodiment 2
[0289] Embodiment 2: the construction of the nucleic acid sequence V022 of encoding giant polyporus (Meripilus giganteus) α-amylase and Athena rotundum glucoamylase CBM
[0290] Primers P003 (SEQ ID NO: 106) and P004 (SEQ ID NO: 107) were used to PCR amplify the polyporus macroporus alpha-amylase gene.
[0291] DNA fragments were recovered from agarose gels using a Qiagen gel extraction kit. The resulting purified fragment was mixed with the vector pLA1 in which the catalytic domain encoding the A. niger α-amylase was excised by digestion with an appropriate restriction endonuclease. The mixed solution was introduced into Saccharomyces cerevisiae to construct expression plasmid pLAV022 by in vivo recombination.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com