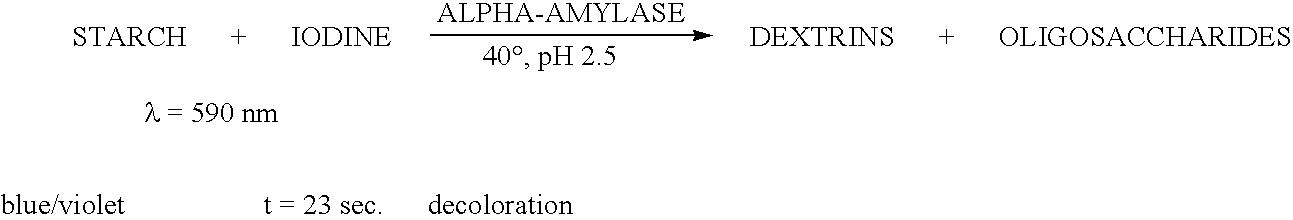
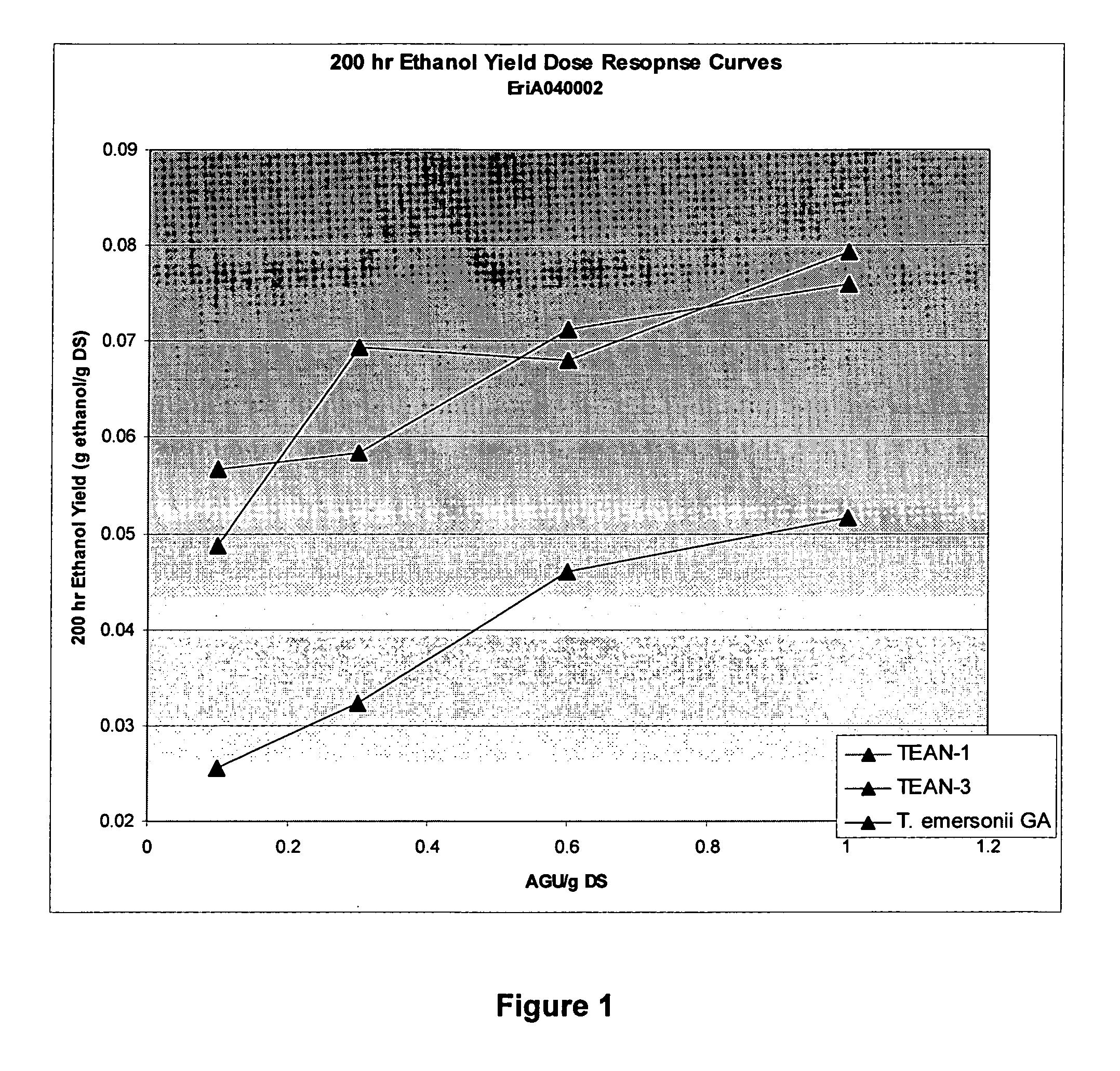
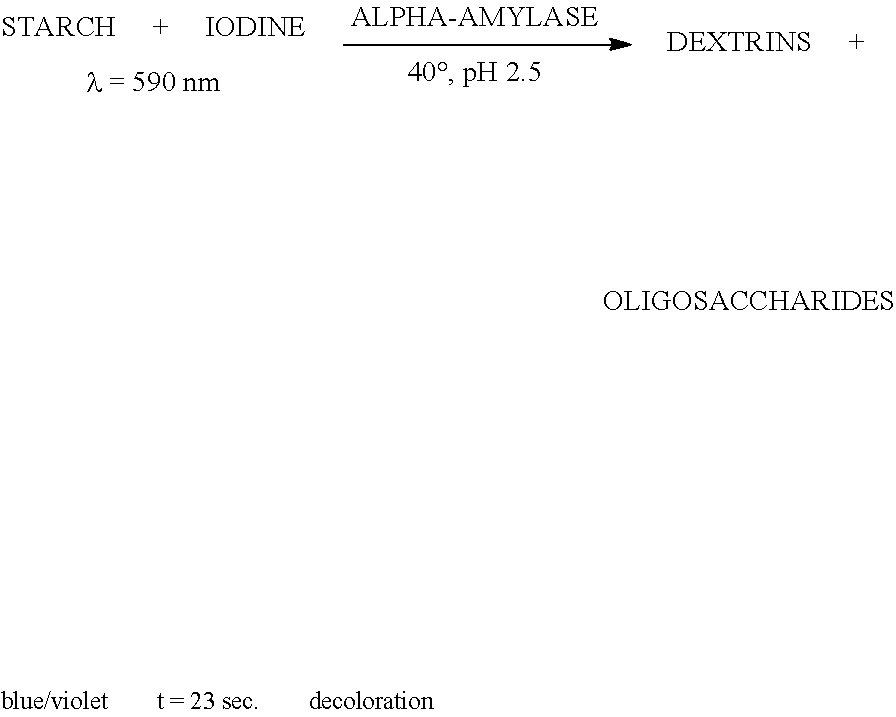
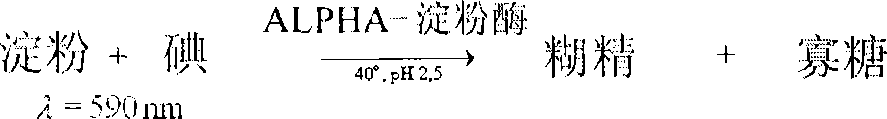Patents
Literature
45 results about "Carbohydrate-binding module" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes (for example glycoside hydrolases). The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains. CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently (June 2011) 64 families of CBM in the CAZy database.
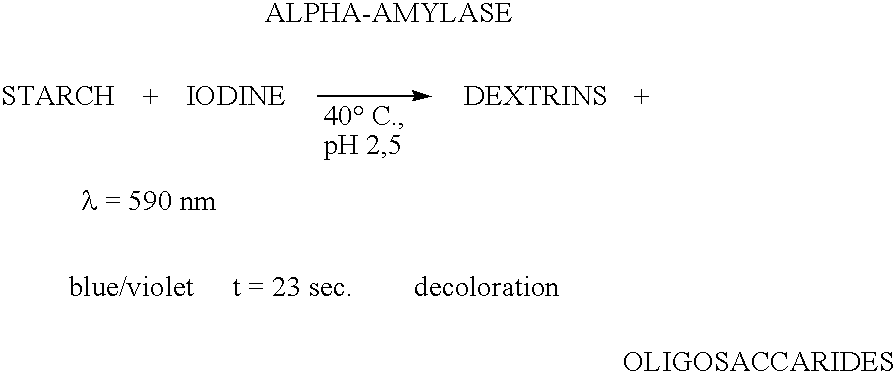
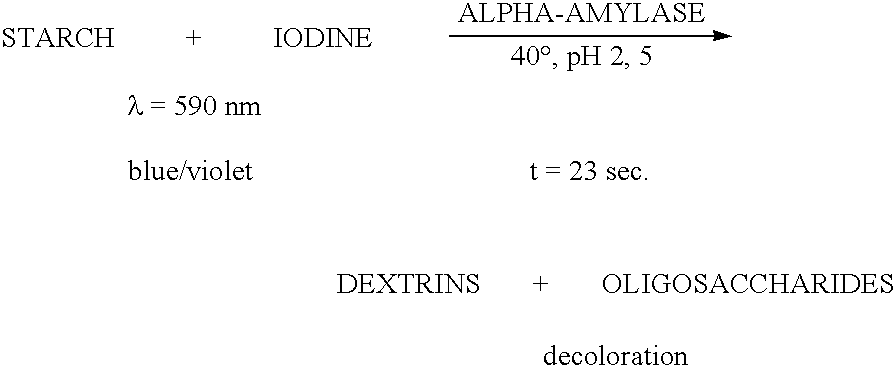
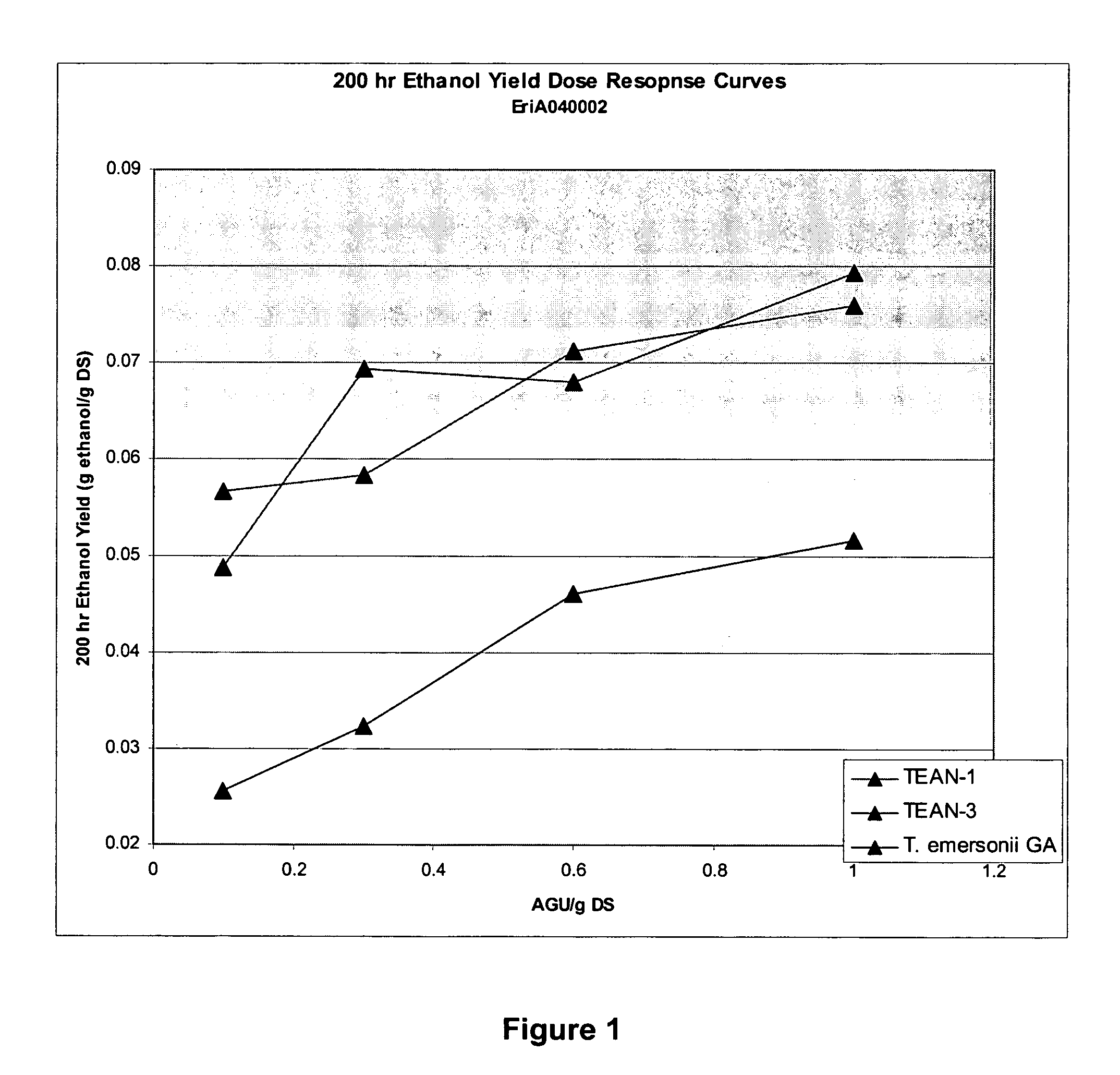
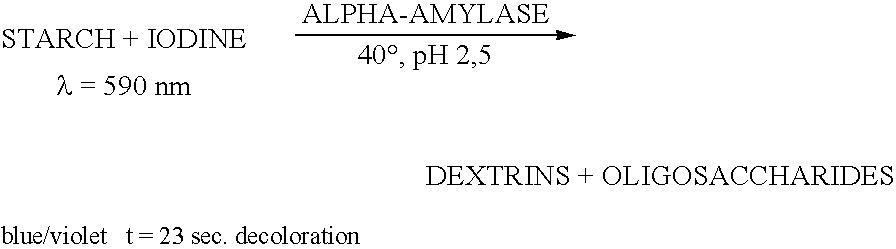
Enzymes for starch processing
InactiveUS20050054071A1Increased acid alpha-amylase activityImprove enzyme stabilitySugar derivativesBacteriaBiotechnologyCarbohydrate-binding protein
The present invention relates to a hybrid enzyme comprising carbohydrate-binding module amino acid sequence and a fungal alpha-amylase amino acid sequence and to a variant of a fungal wild type enzyme comprising a carbohydrate-binding module and an alpha-amylase catalytic module. The invention also relates to the use of the hybrid enzyme or the variant in starch liquefaction.
Owner:NOVOZYMES AS +1
Enzymes for starch processing
InactiveUS20060148054A1Good curative effectActivity be alteredFungiSugar derivativesCarbohydrate-binding proteinAlpha-amylase
The present invention relates to polypeptides comprising a carbohydrate-binding module amino acid sequence and,an alpha-amylase amino acid sequence as well as to the application of such polypeptides.
Owner:NOVOZYMES AS +1
Hybrid enzymes
The present invention relates to a hybrid enzyme comprising at least one carbohydrate-binding module amino acid sequence and at least the catalytic module of a glucoamylase amino acid sequence. The invention also relates to the use of the hybrid enzyme in starch processing and especially ethanol production.
Owner:NOVOZYMES AS +1
Hybrid enzymes
InactiveUS20060257984A1FungiAntibody mimetics/scaffoldsCarbohydrate-binding proteinCompound (substance)
The present invention relates to a hybrid enzyme comprising at least one carbohydrate-binding module amino acid sequence and at least the catalytic module of a glucoamylase amino acid sequence. The invention also relates to the use of the hybrid enzyme in starch processing and especially ethanol production.
Owner:NOVOZYMES AS +1
Enzymes for starch processing
The present invention relates to polypeptides having glucoamylase activity and isolated polynucleotides encoding said polypeptides. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the polynucleotides as well as methods for producing and using the polypeptides. The invention also relates to the composition comprising a glucoamylase of the invention as well as the use such compositions for starch conversion processes, brewing, including processes for producing fermentation products or syrups.
Owner:NOVOZYMES AS +1
Enzymes for starch processing
ActiveUS20140127753A1Good curative effectActivity be alteredAntibody mimetics/scaffoldsPolypeptide with affinity tagCarbohydrate-binding proteinAlpha-amylase
The present invention relates to polypeptides comprising a carbohydrate-binding module amino acid sequence and an alpha-amylase amino acid sequence as well as to the application of such polypeptides.
Owner:NOVOZYMES AS +1
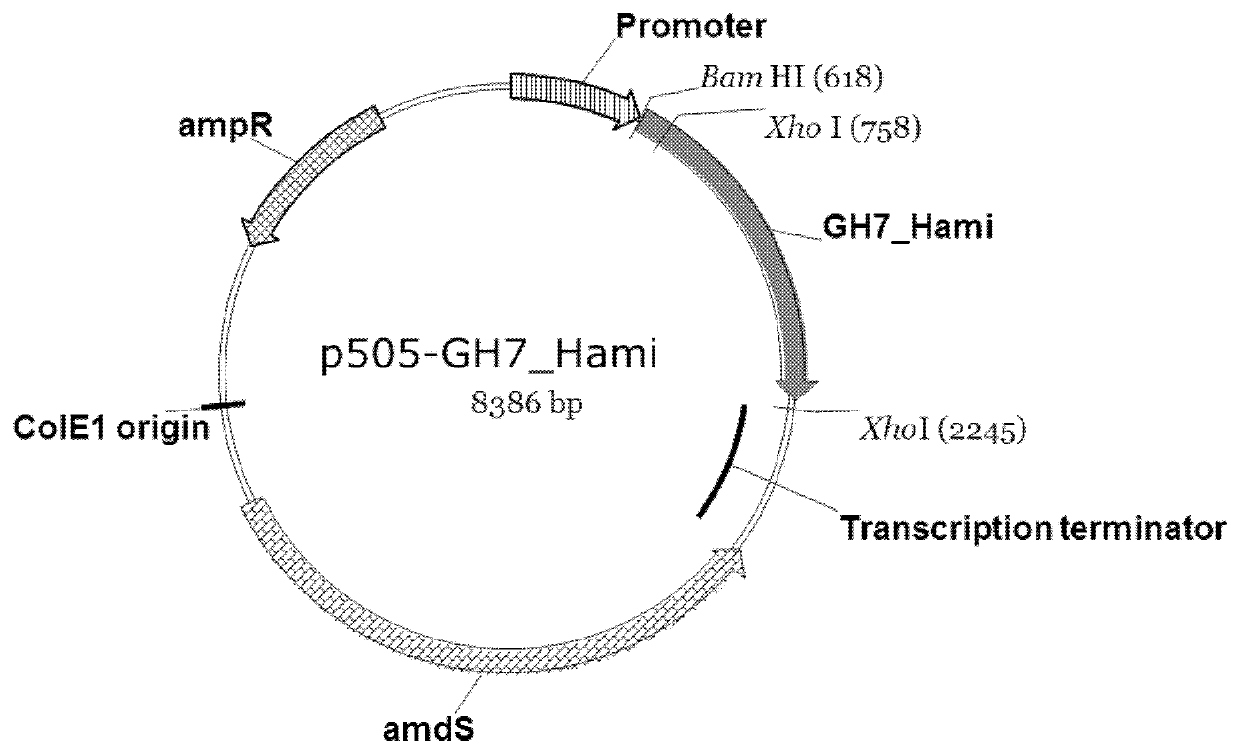
Method and DNA constructs for increasing the production level of carbohydrate degrading enzymes in filamentous fungi
ActiveUS20060014247A1Increase production levelsEasy constructionFungiSugar derivativesBiotechnologyFungal microorganisms
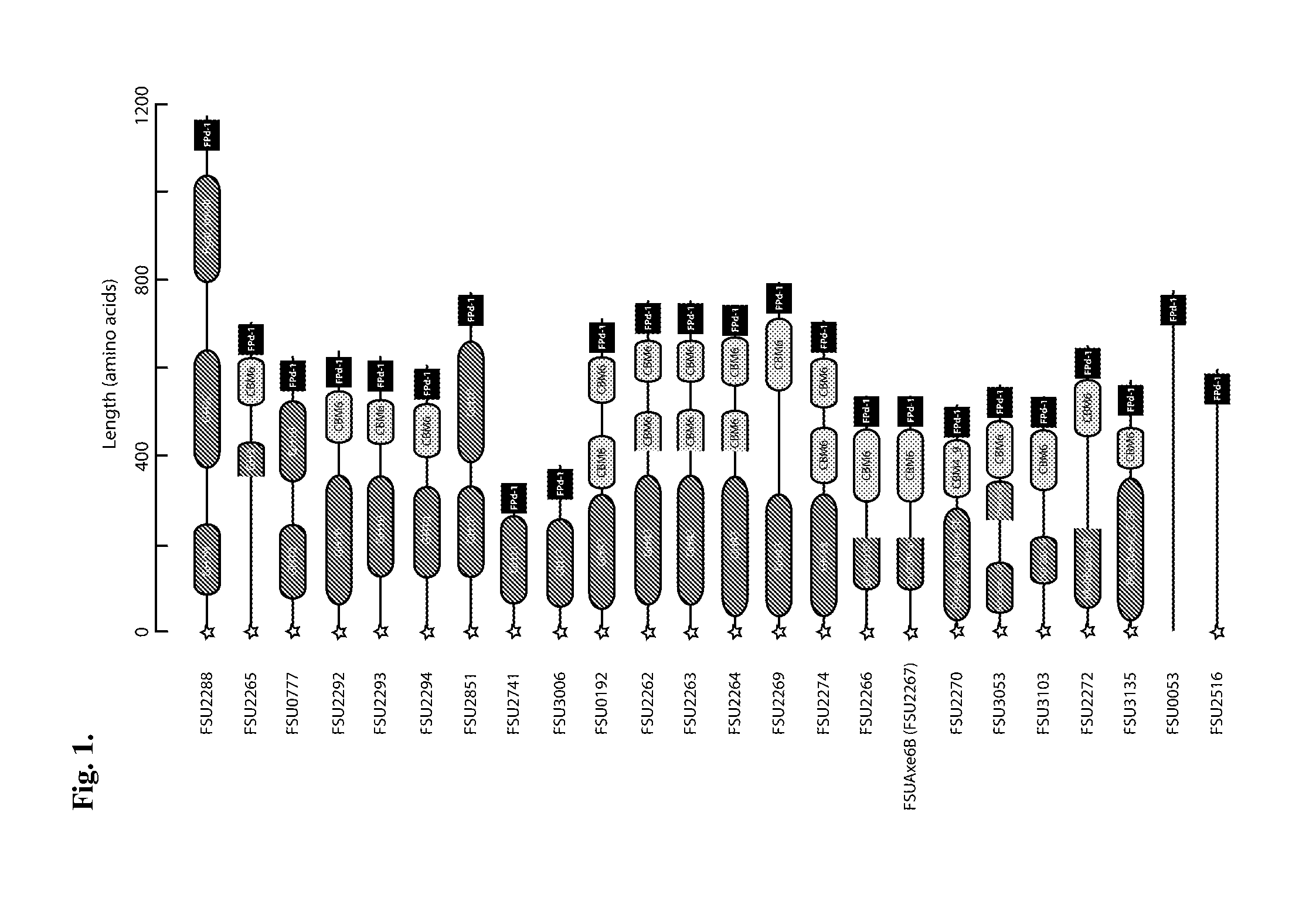
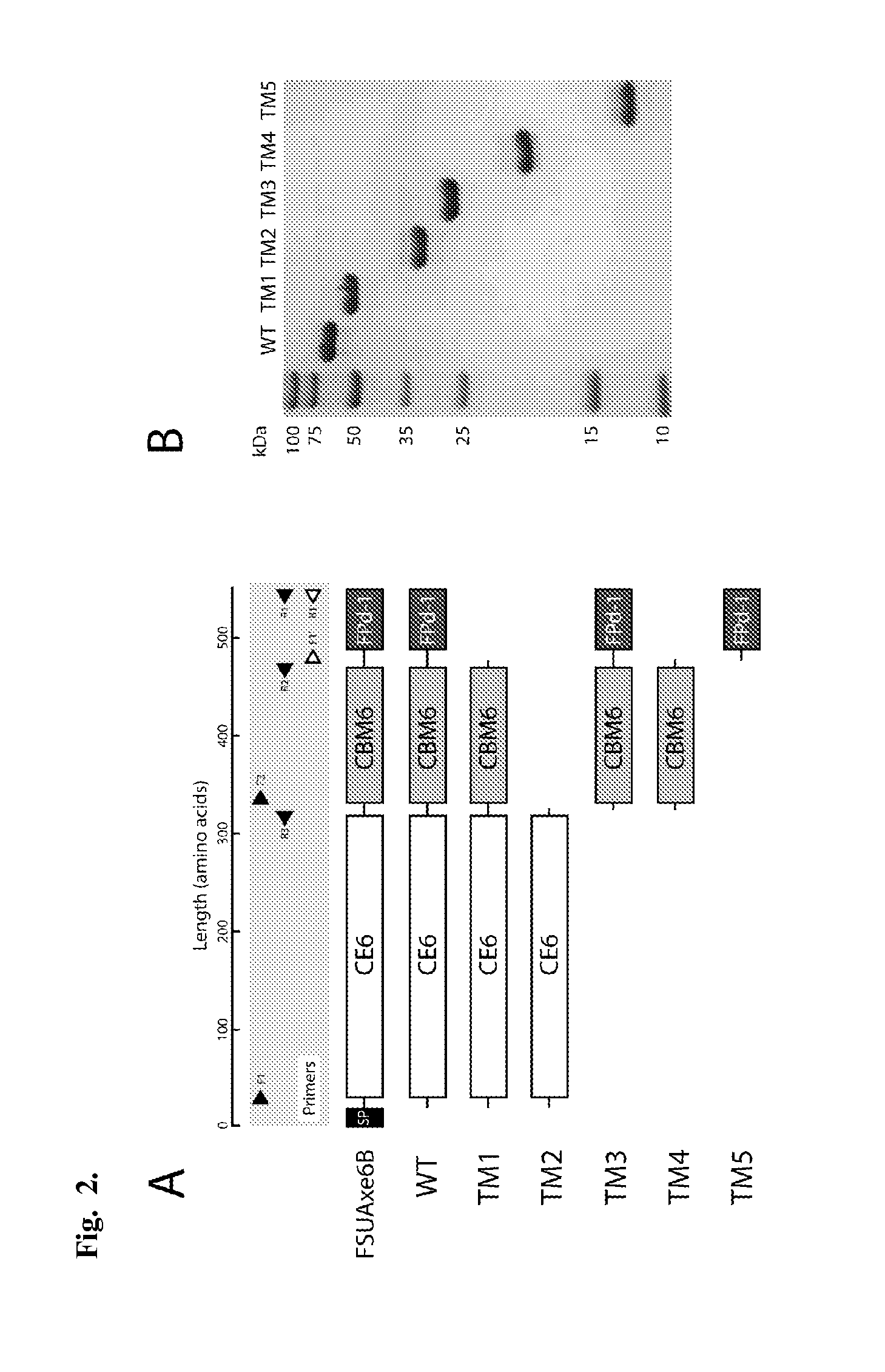
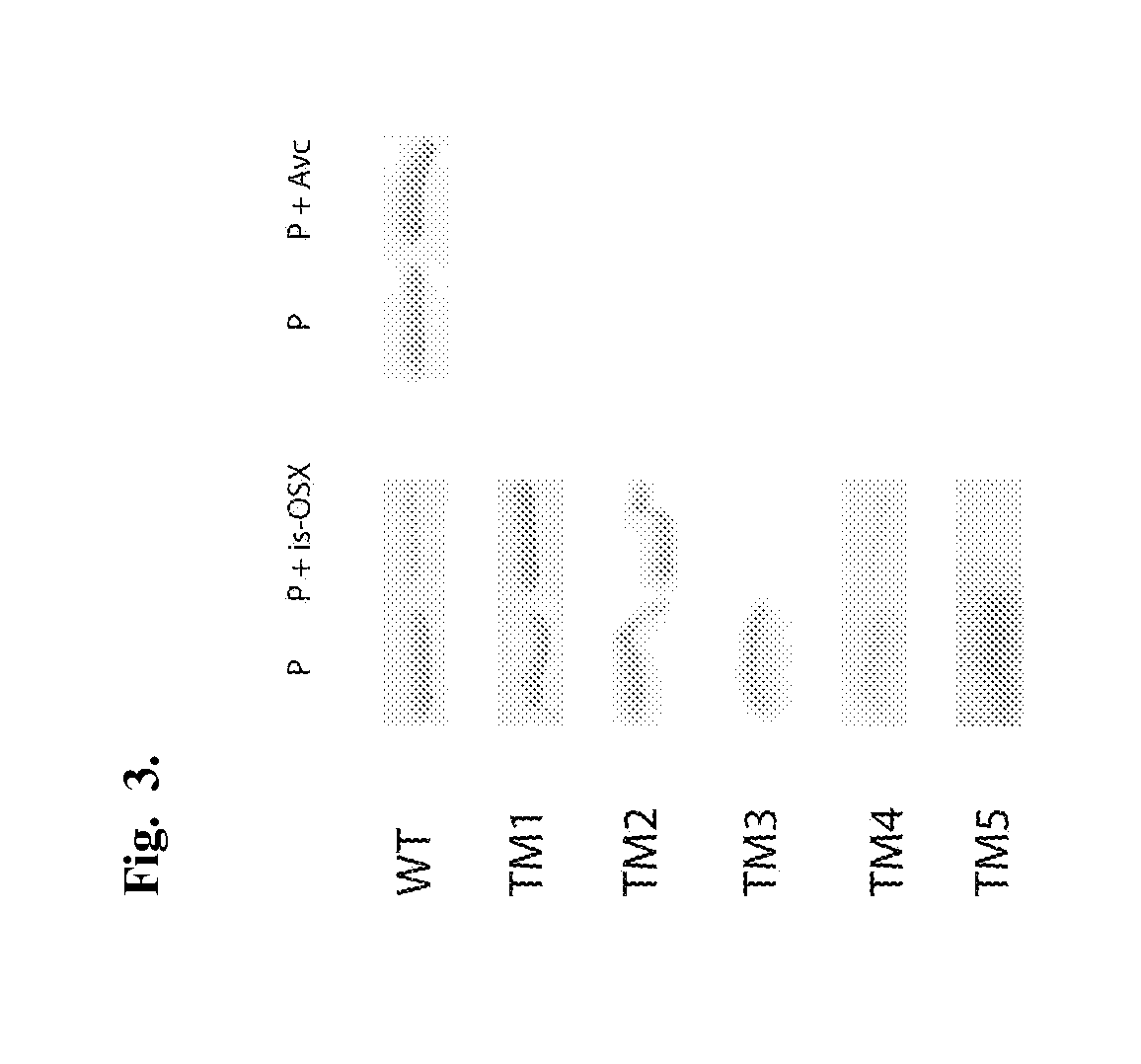
The invention is related to a method and DNA constructs for obtaining in a filamentous fungus host a higher production level of a carbohydrate degrading (CD) enzyme, having in its original state a catalytic module (CAT) and a carbohydrate binding module (CBM) separated by a linker region. The DNA construct comprising a truncated actinomycetes, preferably Nonomuraea flexuosa (NJ) derived DNA sequence encoding a truncated form of the CD enzyme, for example Nf Xyn11A, Nf Xyn10A, and is introduced into a filamentous fungal host. Said CD enzyme contains the catalytically active region of CAT but lacks part or all of the CBM, or all of the CBM and part or all of the linker region and is expressed and secreted under the control of regulatory sequences comprising at least a signal sequence, but also promoters, terminators and DNA sequences encoding a secretable carrier protein or domains thereof, preferably originating from filamentous fungi are included. The production level obtained with DNA sequence having the shortened DNA sequence encoding the truncated form of the CD enzyme is higher than the production level obtained with DNA construct encoding the corresponding full length CD enzyme.
Owner:AB ENZYMES OY
Cellulose immobilized cis epoxy succinic acid hydrolase and method for preparing tartaric acid
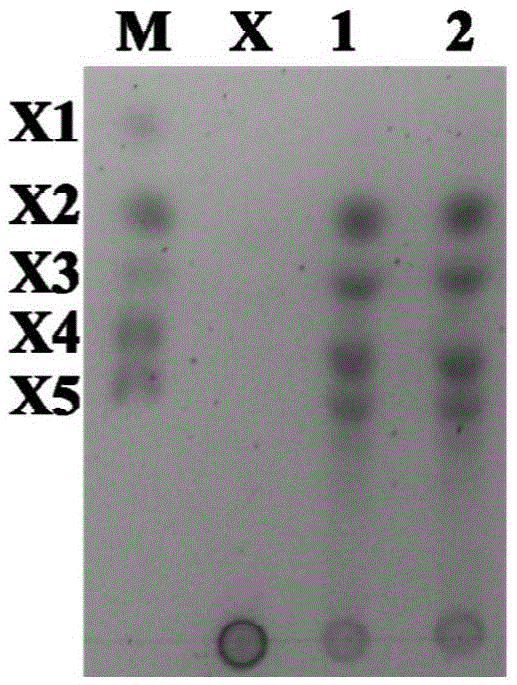
InactiveCN102703419ALow priceWide variety of sourcesOn/in organic carrierFermentationCelluloseEscherichia coli
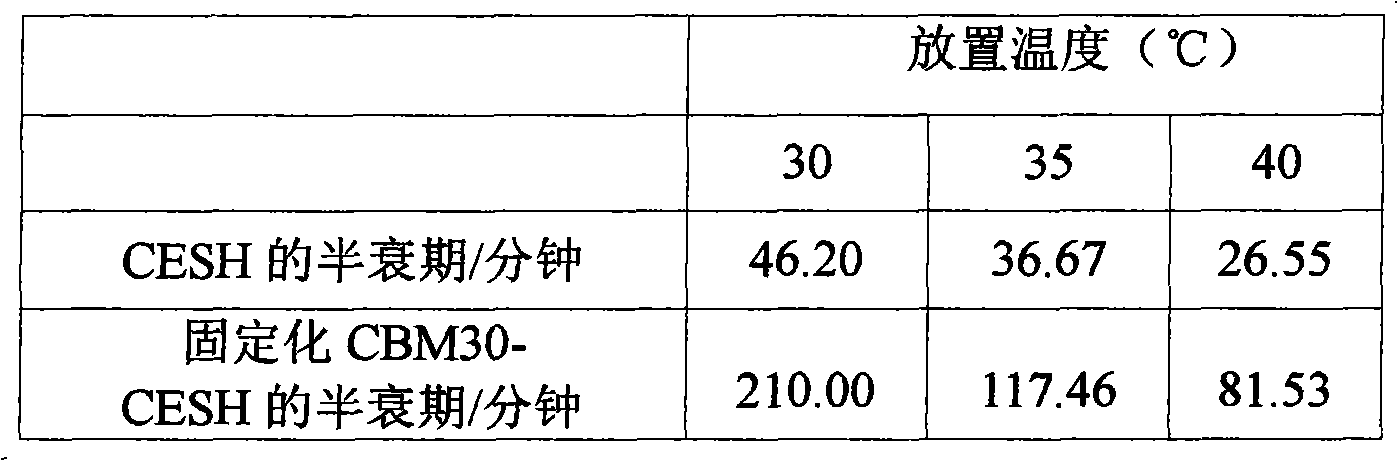
The invention provides a method of cellulose immobilized cis epoxy succinic acid hydrolase. The method comprises the following steps: fusing gene coding cis epoxy succinic acid hydrolase and carbohydrate binding module CMB30, efficiently expressing the fused gene in Escherichia coli, and then carrying out purification and immobilization on fused enzyme in crude enzyme liquid by adopting the cellulose. The invention also provides a method for catalyzing and hydrolyzing epoxy succinic acid to convert into L-tartaric acid or D-tartaric acid by adopting the cellulose immobilized cis epoxy succinic acid hydrolase as biocatalyst. The method has the beneficial effects that: not only is the stability of enzyme obviously improved, but also the cellulose used as purified and fixed substrate is low in price and easy to get by adopting the cellulose immobilized cis epoxy succinic acid hydrolase, and has excellent economic prospect. The method for preparing tartaric acid by adopting the cellulose immobilized cis epoxy succinic acid hydrolase has the advantages of reaction specificity, high product purity, high reaction efficiency and the like.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Polypeptides Having Mannanase Activity and Polynucleotides Encoding Same
InactiveUS20170183643A1Convenient ligationAvoid mistakesOxidoreductasesDetergent compounding agentsMannanase activityNucleotide
The present invention relates to polypeptides having mannanase activity, catalytic domains, and carbohydrate binding modules, and polynucleotides encoding the polypeptides, catalytic domains, and carbohydrate binding modules. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the polynucleotides as well as methods of producing and using the polypeptides, catalytic domains, and carbohydrate binding modules.
Owner:NOVOZYMES AS
Enzymes for starch processing
ActiveUS8841091B2Good curative effectActivity be alteredSugar derivativesAntibody mimetics/scaffoldsCarbohydrate-binding proteinAlpha-amylase
The present invention relates to polypeptides comprising a carbohydrate-binding module amino acid sequence and an alpha-amylase amino acid sequence as well as to the application of such polypeptides.
Owner:NOVOZYMES AS +1
Enzymes for starch processing
ActiveUS20110104759A1Good curative effectActivity be alteredSugar derivativesAntibody mimetics/scaffoldsCarbohydrate-binding proteinAlpha-amylase
The present invention relates to polypeptides comprising a carbohydrate-binding module amino acid sequence and an alpha-amylase amino acid sequence as well as to the application of such polypeptides.
Owner:NOVOZYMES AS +1
Enzymes for Starch Processing
ActiveUS20140370553A1Improve efficacyActivity be alteredAntibody mimetics/scaffoldsPolypeptide with affinity tagAmylaseCarbohydrate-binding module
The present invention relates to polypeptides comprising a carbohydrate-binding module amino acid sequence and an alpha-amylase amino acid sequence as well as to the application of such polypeptides.
Owner:NOVOZYMES AS +1
A high-temperature resisting neutral xylanase gene and engineering bacteria containing the gene
InactiveCN102191260AImprove stabilityBacteriaMicroorganism based processesAnimal ForagingConserved sequence
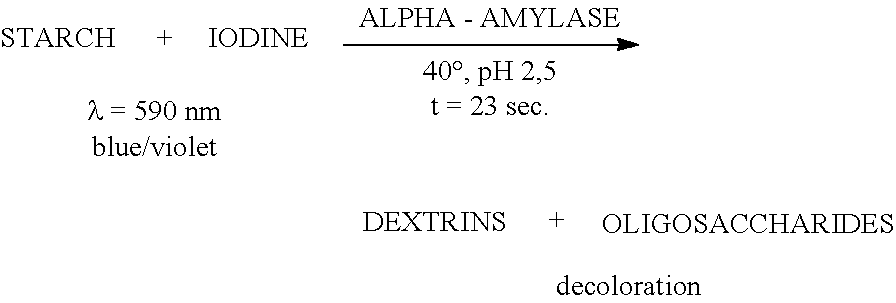
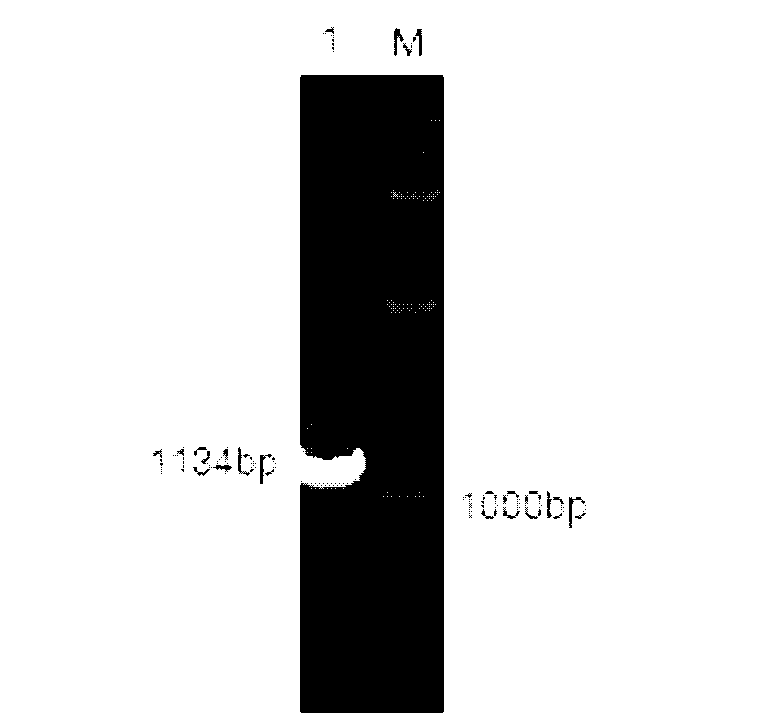
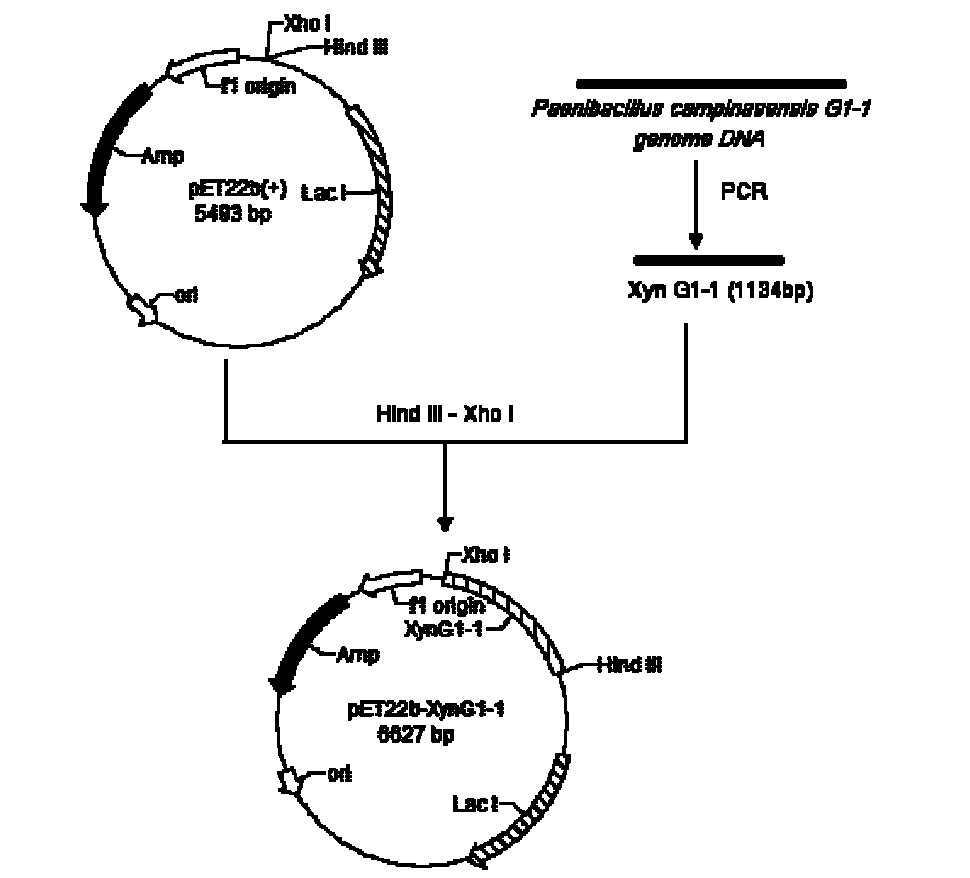
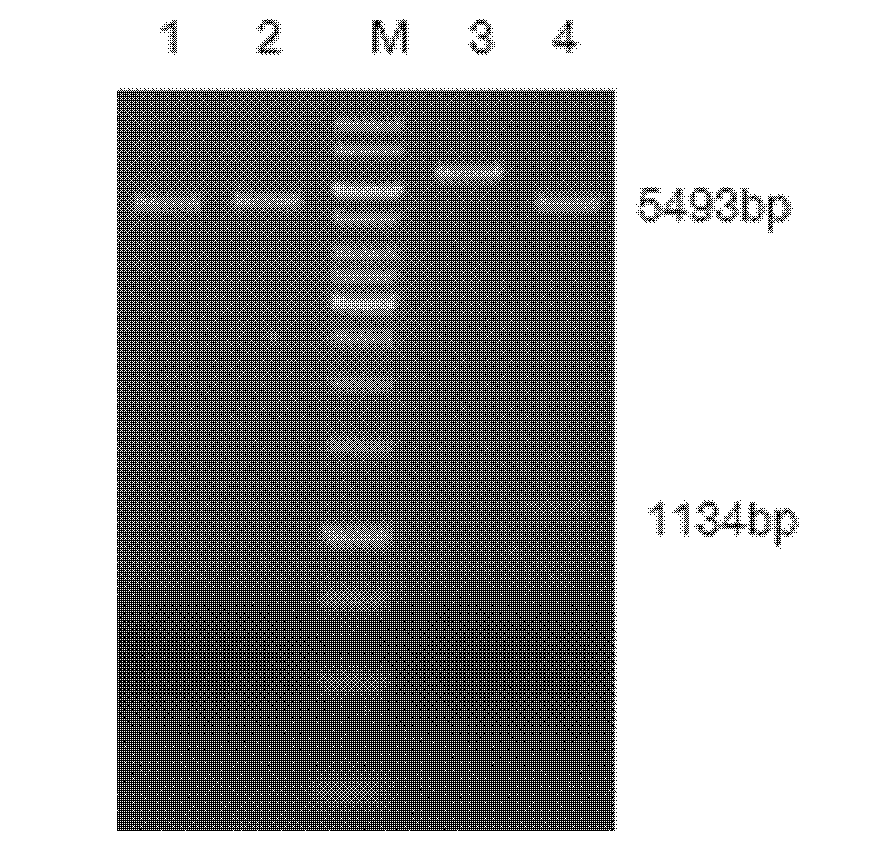
The invention relates to a high-temperature resisting neutral xylanase gene and engineering bacteria containing the gene. A gene sequence refers to a sequence 1, the whole length of the gene is 1134 bp and the gene is named as XynG1-1, the gene codes 377 amino acids, molecular weight of the gene is 41267 Da, wherein former 39 amino acids are signal peptide of the gene, the 49th to 235th amino acids are a conserved sequence of a glucosides hydrolase GH11 family, the 263rd to 377th amino acids are a conserved sequence of a carbohydrate-binding module CBM6 family. According to the invention, the recombinase obtained by fermenting the engineering bacteria has optimum temperature of 60 DEG C and optimum pH value of 7.0, heat is preserved at a pH value of 7.0 and temperature of 70 DEG C for 1 hour, the relative enzyme activity is more than 65%, and heat is preserved at a pH value of 9 and temperature of 60 DEG C for 1 hour, the relative enzyme activity is more than 60%. The xylanase of the present invention has the characteristic of good stability under high temperature, and can be applied to a plurality of industrial fields such as papermaking, foodstuff and forage, etc. The present invention has wide application prospect.
Owner:TIANJIN UNIV OF SCI & TECH
Polymer Probes And Methods
ActiveUS20170115267A1Effective wayQuick checkPeptide-nucleic acidsBiological material analysisCarbohydrate-binding proteinCarbohydrate-binding module
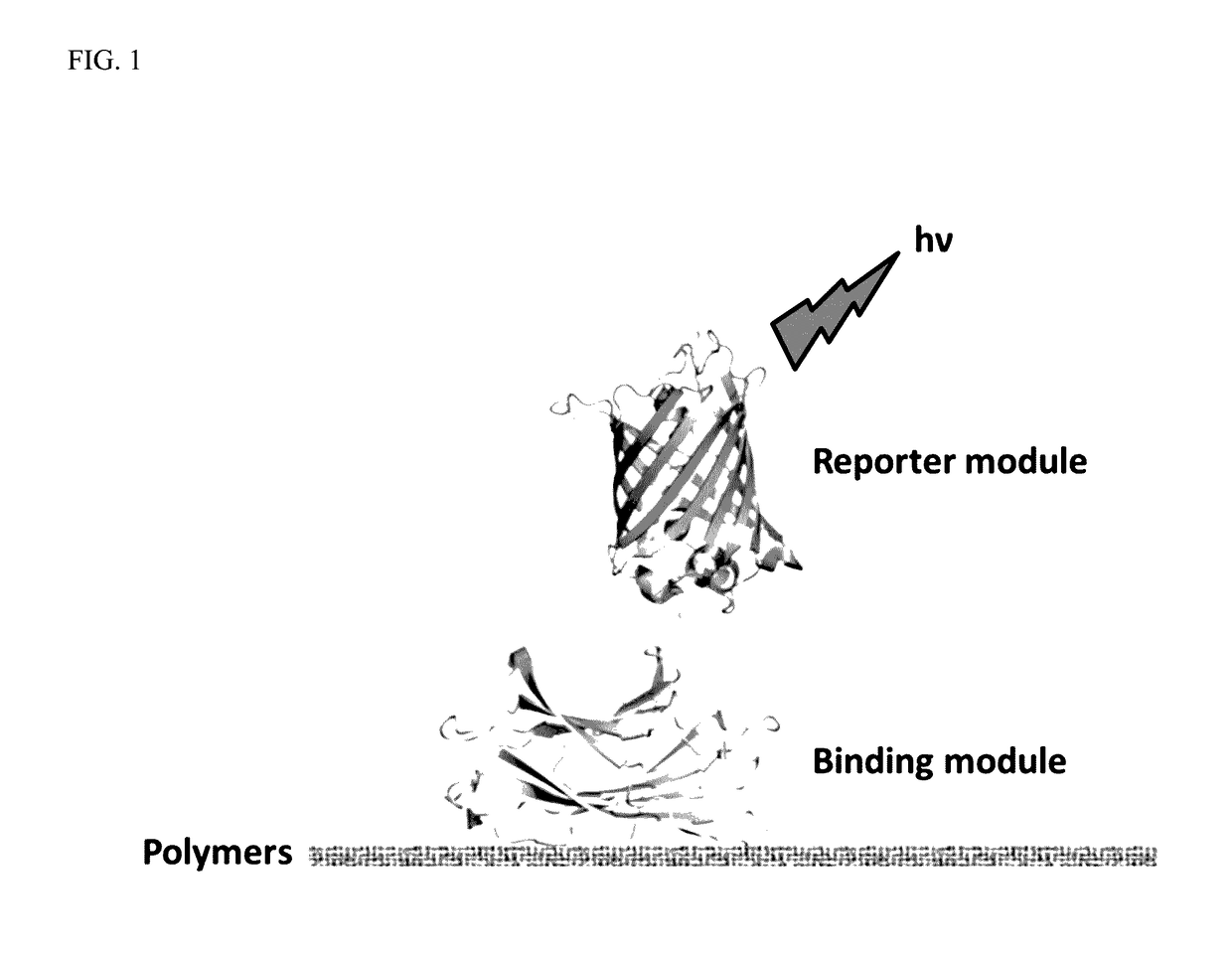
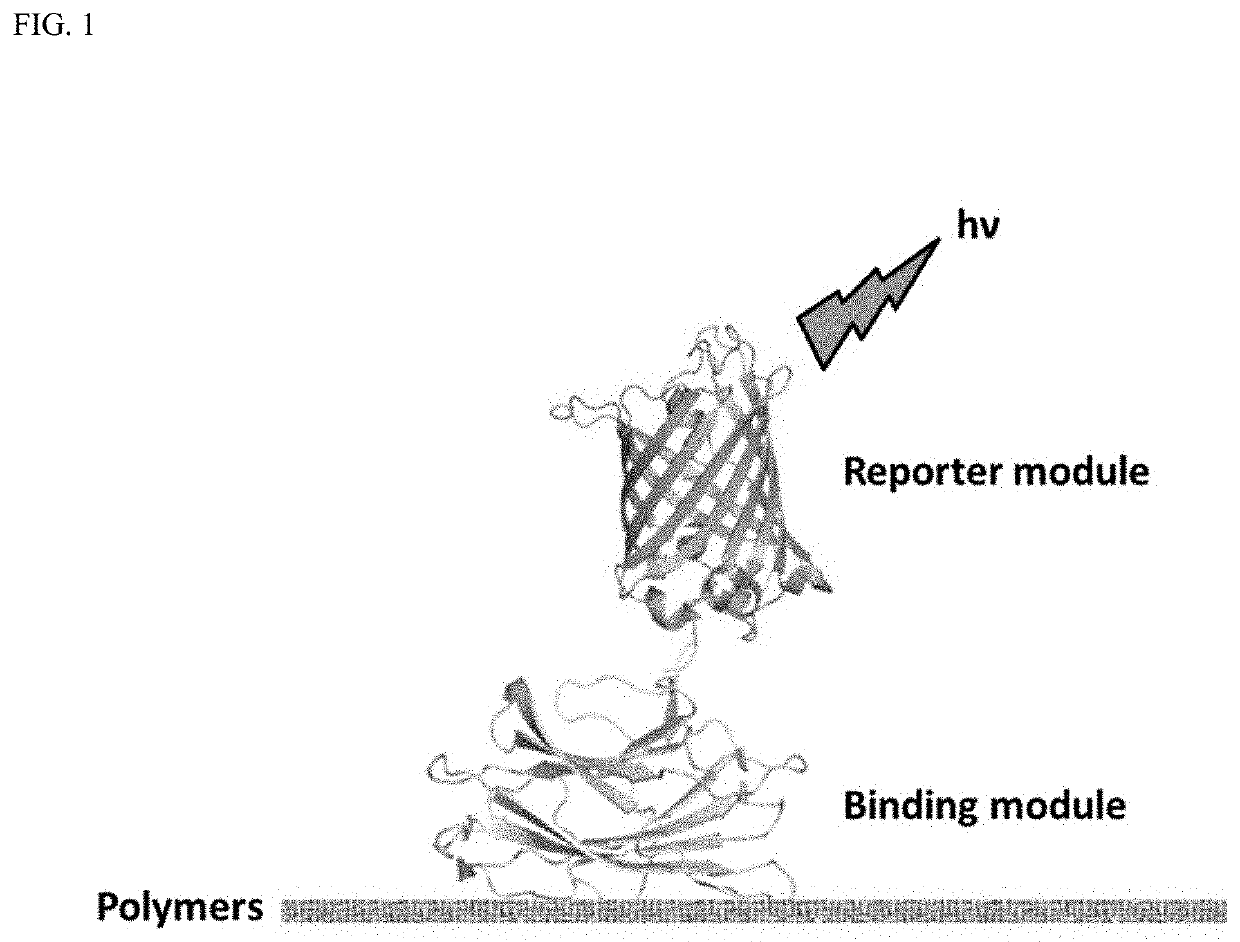
A polymer detection probe is provided that includes a binding module that specifically binds to at least one polymer and a reporter module that is spectroscopically detectable. The binding module can be a carbohydrate-binding module (CBM). The reporter module can be a fluorescent protein. A complex is provided that includes a probe specifically bound to a pulp or paper product including at least one surface available lignocellulosic polymer. A pulp or paper product is provided that includes at least one surface available lignocellulosic polymer and at least one probe bound thereto. Methods are provided that employ a lignocellulosic probe. A method of detecting a lignocellulosic polymer or other type of polymer is provided. A method of determining the effectiveness of an industrial treatment on pulp or a paper product is also provided. A method of determining a physical property of pulp or a paper product is further provided.
Owner:BUCKMAN LAB INT INC +1
Method and DNA constructs for increasing the production level of carbohydrate degrading enzymes in filamentous fungi
The invention is related to a method and DNA constructs for obtaining in a filamentous fungus host a higher production level of a carbohydrate degrading (CD) enzyme, having in its original state a catalytic module (CAT) and a carbohydrate binding module (CBM) separated by a linker region. The DNA construct comprising a truncated actinomycetes, preferably Nonomuraea flexuosa (NJ) derived DNA sequence encoding a truncated form of the CD enzyme, for example Nf Xyn11A, Nf Xyn10A, and is introduced into a filamentous fungal host. Said CD enzyme contains the catalytically active region of CAT but lacks part or all of the CBM, or all of the CBM and part or all of the linker region and is expressed and secreted under the control of regulatory sequences comprising at least a signal sequence, but also promoters, terminators and DNA sequences encoding a secretable carrier protein or domains thereof, preferably originating from filamentous fungi are included. The production level obtained with DNA sequence having the shortened DNA sequence encoding the truncated form of the CD enzyme is higher than the production level obtained with DNA construct encoding the corresponding full length CD enzyme.
Owner:AB ENZYMES OY
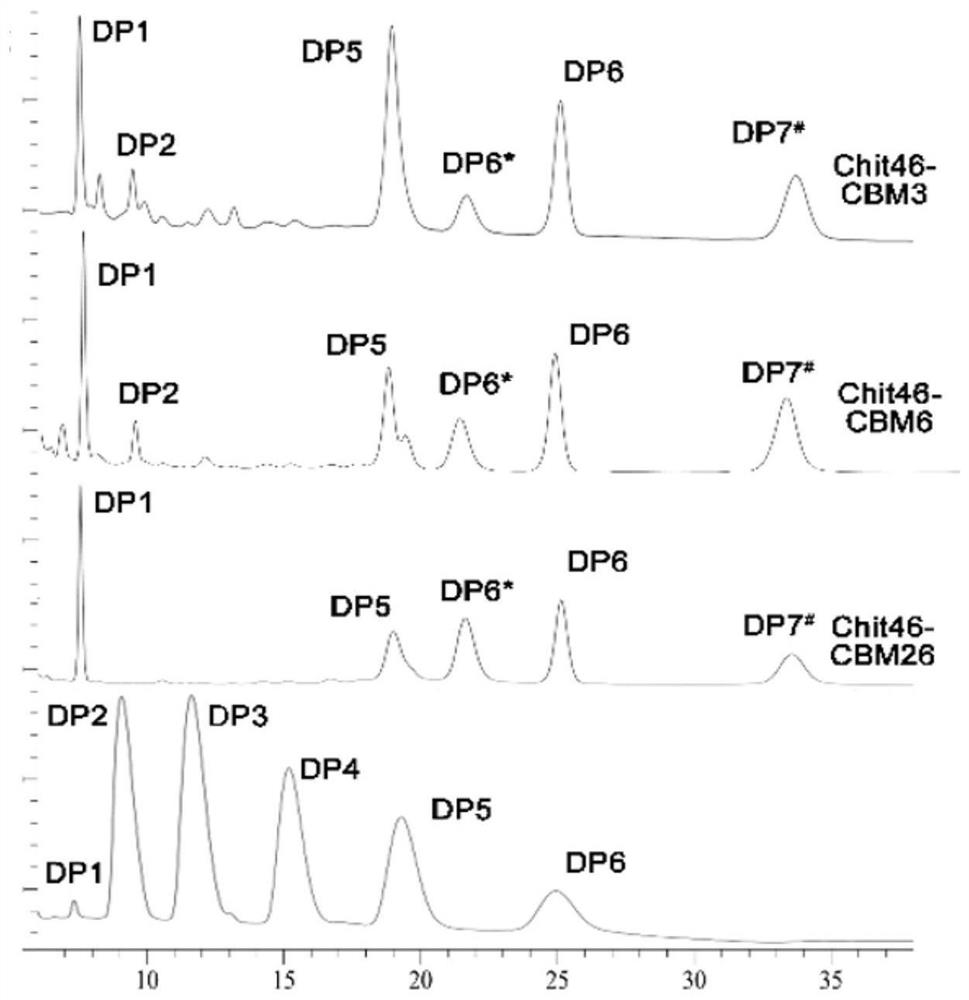
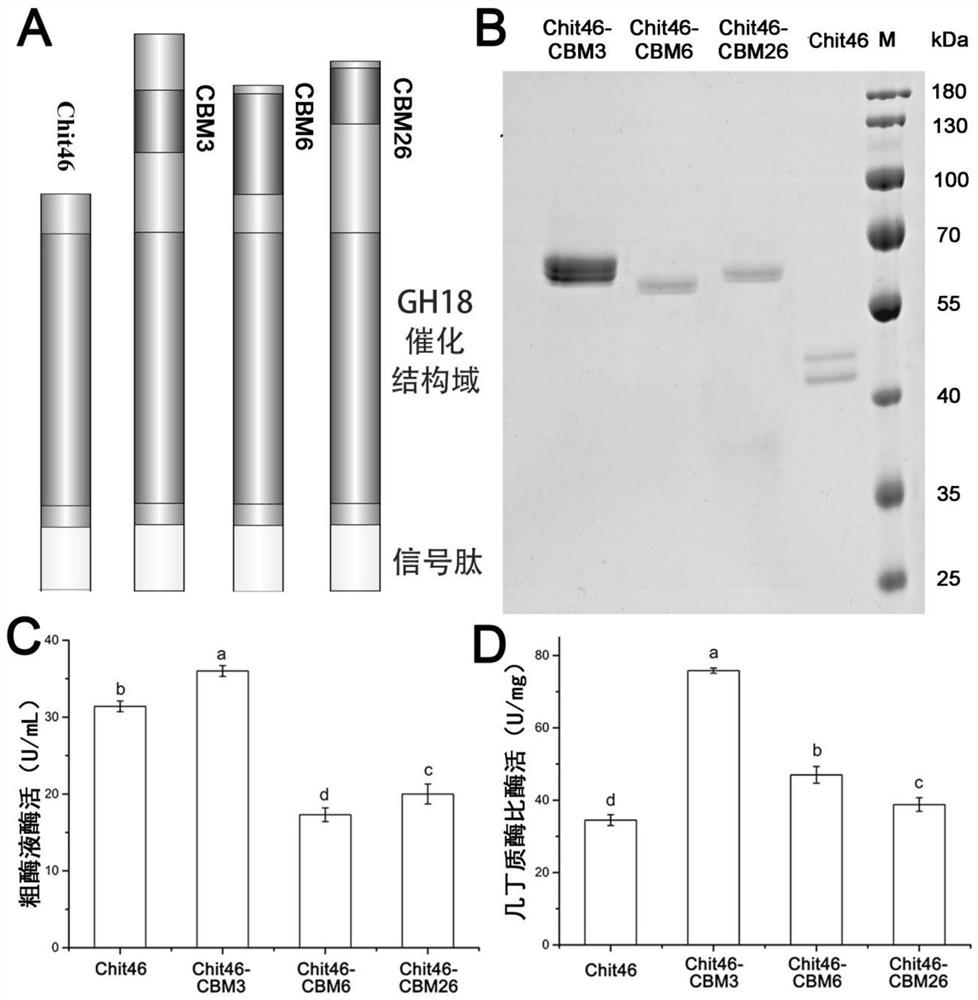
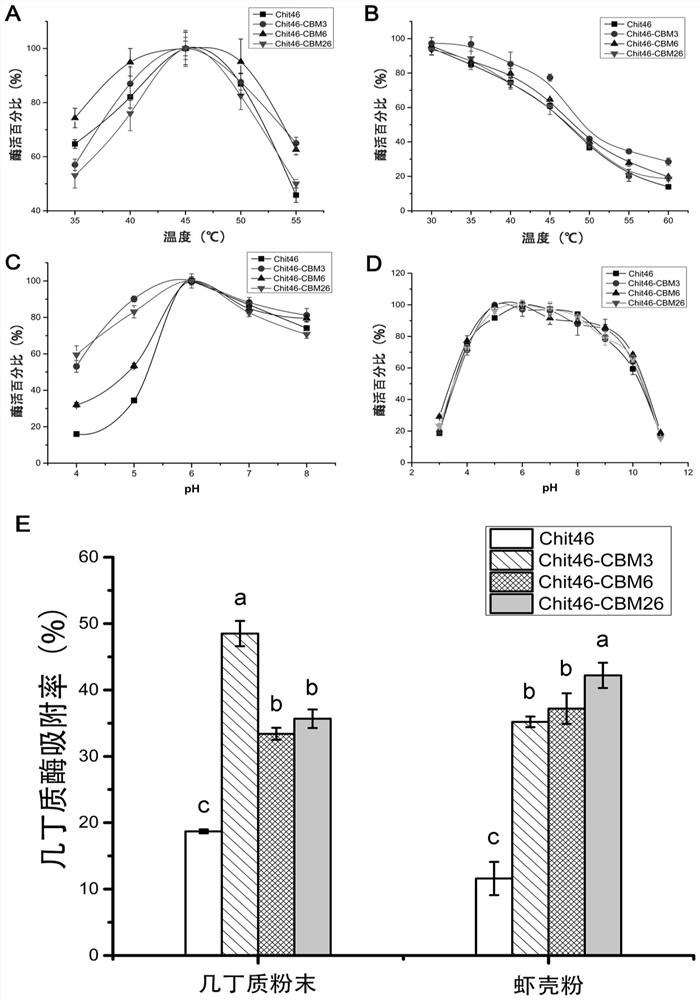
Chitinase fused with carbohydrate binding module, preparation method for chitinase and application of chitinase
ActiveCN111944787AImprove recycling effectAvoid using effectsFungiAntibody mimetics/scaffoldsCarbohydrate-binding proteinChitinase
The invention provides chitinase fused with a carbohydrate binding module, a preparation method for the chitinase and application of the chitinase, and belongs to the technical field of biological engineering. The chitinase fused with the carbohydrate binding module comprises fusion chitinase formed by series connection of the carbohydrate binding module with a connecting peptide and a C end of chitinase Chit46. It is the first time for the invention to successfully apply the chitinase Chit46-CBM3, Chit46-CBM6 and Chit46-CBM26 fused with the carbohydrate binding module (CBM) to shrimp shell waste recovery. After the CBM is fused, the chitin in shrimp shells can be directly hydrolyzed by the fusion chitinase, and thus, the use of a strong alkali reagent and a complex pretreatment step in atraditional process are avoided.
Owner:SOUTH CHINA UNIV OF TECH
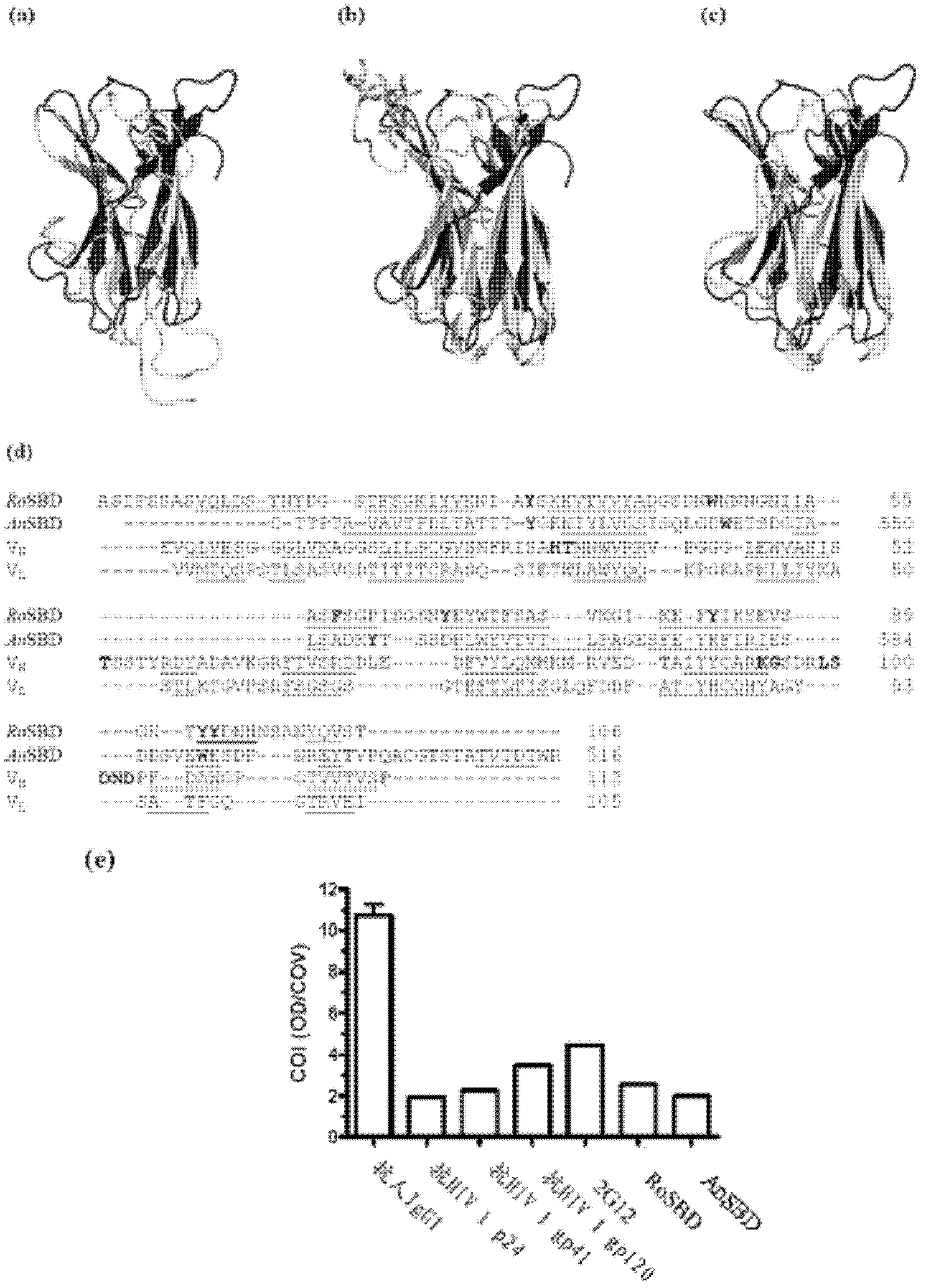
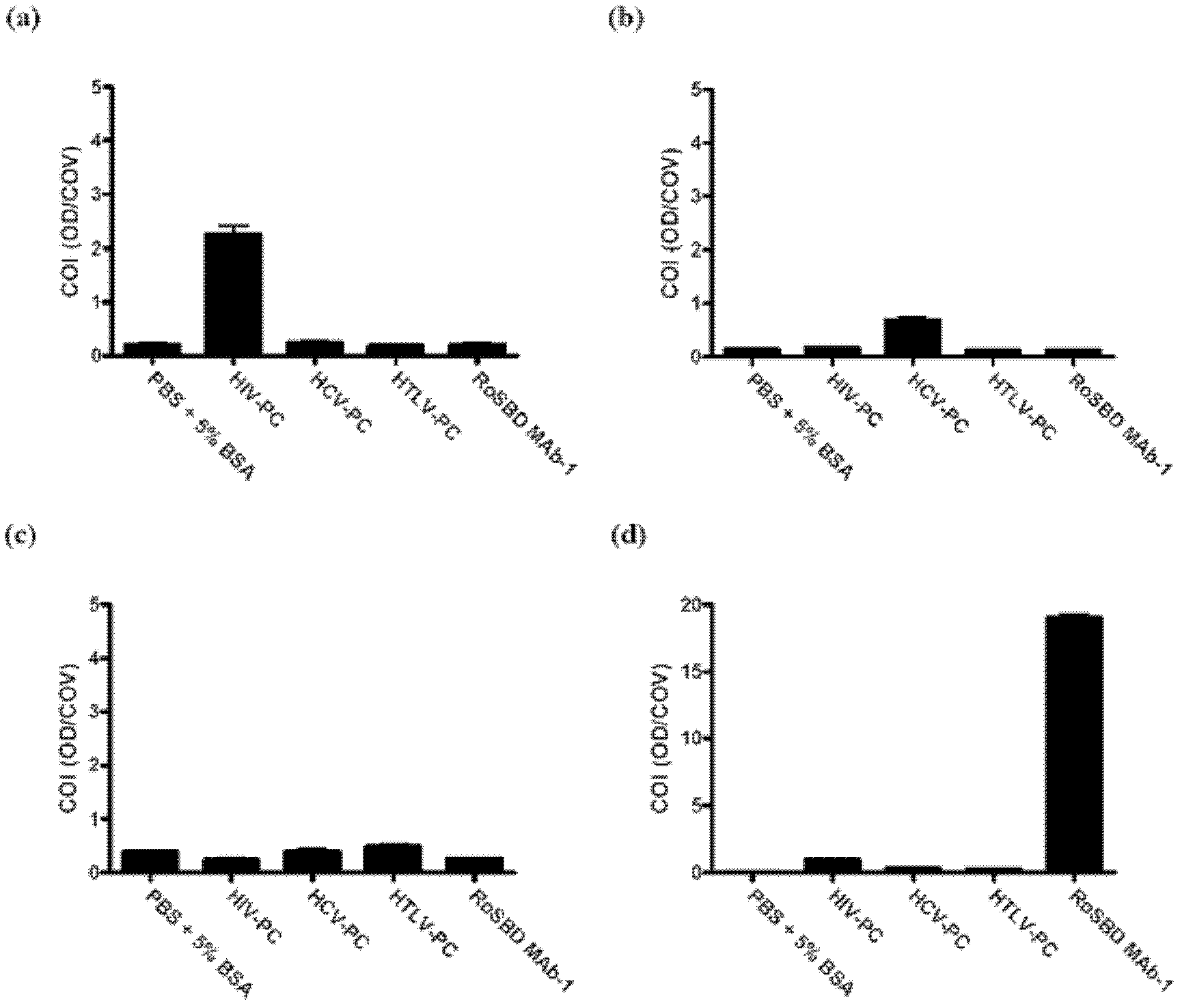
Carbohydrate binding module and use thereof
InactiveCN103097411AMicrobiological testing/measurementImmunoglobulins against virusesEpitopeCarbohydrate-binding protein
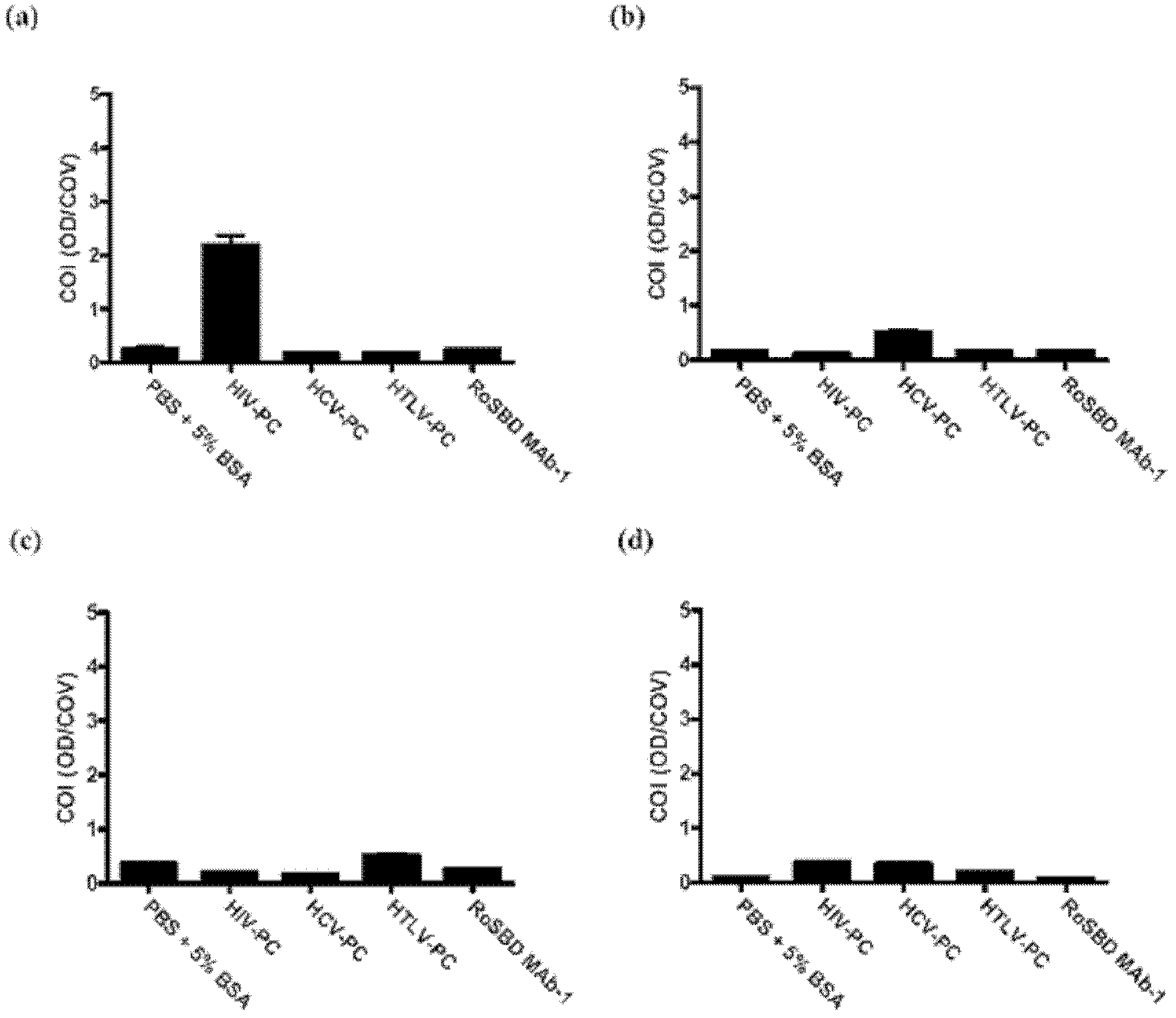
The present invention provides a method of inhibiting HIV infection of a subject, comprising administering to the subject an effective amount of an antibody mimetic of carbohydrate binding module (CBM) which specifically binds to an epitope on HIV glycoprotein. The present invention also provides a pharmaceutical composition comprising a therapeutically or prophylactically effective amount of an antibody mimetic of carbohydrate binding module and a pharmaceutically acceptable carrier. The present invention also provides a method of inhibiting HIV infection of the subject preventionally or therapically comprising administering to the subject an effective amount of an antibody mimetic of carbohydrate binding module (CBM) which specifically binds to an epitope on HIV glycoprotein
Owner:张大慈
Feruloyl esterase as well as mutants and application thereof
ActiveCN112980812AIncrease enzyme activityHigh activityHydrolasesFood processingDisulfide bondingSide chain
The invention discloses feruloyl esterase as well as mutants and application thereof. The feruloyl esterase has an amino acid sequence as shown in SEQ ID No. 1, SEQ ID No. 2 or SEQ ID No. 3, the feruloyl esterase is stable in property, the reaction pH is 6-8, and the reaction temperature is 37-54 DEG C. On the basis of the amino acid sequence of the feruloyl esterase, three feruloyl esterase mutants N.1-300, N.7-16 and N.9-98 are obtained by increasing the number of disulfide bonds in the feruloyl esterase, changing side chain groups of a carbohydrate binding module of the feruloyl esterase and changing the charges of a catalytic structural domain, and compared with original feruloyl esterase, the specific enzyme activities of the three mutants are obviously improved. The feruloyl esterase and the mutant obtained by the invention have stable properties, and are beneficial to industrial application of the feruloyl esterase.
Owner:NANJING AGRICULTURAL UNIVERSITY
Feruloyl esterase as well as mutant N.7-16 and application thereof
ActiveCN114774388AIncrease enzyme activityHigh activityHydrolasesFood processingDisulfide bondingSide chain
The invention discloses feruloyl esterase, a mutant N.7-16 of the feruloyl esterase and application of the feruloyl esterase. The feruloyl esterase has an amino acid sequence as shown in SEQ ID No.2 and is stable in property, the reaction pH is 6-8, and the reaction temperature is 37-54 DEG C. On the basis of the amino acid sequence of the feruloyl esterase, the feruloyl esterase mutant N.7-16 is obtained by increasing the number of disulfide bonds in the feruloyl esterase, changing side chain groups of a carbohydrate binding module and changing the charges of a catalytic structural domain, and compared with original feruloyl esterase, the feruloyl esterase mutant N.7-16 has the advantages that the feruloyl esterase mutant N.7-16 is obtained; the specific enzyme activity of the mutant is obviously improved. The feruloyl esterase and the mutant obtained by the invention have stable properties, and are beneficial to industrial application of the feruloyl esterase.
Owner:NANJING AGRICULTURAL UNIVERSITY
Novel xylanase produced from streptomyces sp. strain hy-14
The present invention relates to a novel xylanase homologous with an enzyme corresponding to a glycoside hydrolase family 10 (GH10) domain and comprising a carbohydrate-binding module family 2 (cellulose-binding module family 2, CBM2) domain, a substrate-binding module, which were isolated from Streptomyces sp. strain HY-14. Particularly, the maximum enzyme activity continues at at least 80% at a pH between 5.0 and 7.5 and the sugar substrates including xylans are significantly degraded in the xylanase, and thus the xylanase can be used as an enzyme in various industrial sectors such as food, feed, and other industries.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Carbohydate binding modules with reduced binding to lignin
ActiveUS9206406B2Reduced binding to ligninPromote recoveryBacteriaPeptide/protein ingredientsCarbohydrate-binding proteinAmino acid substitution
Owner:IOGEN ENERGY CORP
Novel cellobiohydrolase enzymes
Provided are isolated cellobiohydrolases comprising a modified Glycoside Hydrolase (GH) Family 7 catalytic domain, a GH Family 7 catalytic domain and a modified Family carbohydrate binding module (CBM), or both a modified Family 7 catalytic domain and a modified Family 1 CBM. Such isolated cellobiohydrolases exhibit from 45% to about 99.9% amino acid sequence identity to amino acids 1- 436 of SEQ ID NO: 1 or to amino acids 1-438 of SEQ ID NO: 2 and improved activity on process substrates. Also provided are genetic constructs and genetically modified microbes for expressing the isolated cellobiohydrolases, a process for producing the isolated cellobiohydrolases, cellulase enzyme mixtures comprising the isolated cellobiohydrolase and a process for hydrolysing a cellulosic substrate with such cellulase enzyme mixtures.
Owner:IOGEN ENERGY CORP
Multifunctional cellulase and hemicellulase
Owner:WISCONSIN ALUMNI RES FOUND
Carbohydrate binding module with affinity for insoluble xylan
InactiveUS20110237448A1Improve abilitiesPeptide/protein ingredientsHydrolasesOpen reading frameCarbohydrate-binding protein
The present disclosure relates to isolated polynucleotides with two polynucleotide sequences linked within one open reading frame, in which the first polynucleotide sequence encodes a peptide that binds to a carbohydrate. The present disclosure also relates to vectors and genetically modified host cells containing such isolated polynucleotides and polypeptides encoded by such isolated polynucleotides. The present disclosure further relates to methods of increasing the ability of a recombinant protein to bind to a carbohydrate and methods of identifying a protein having an ability to bind to a carbohydrate.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
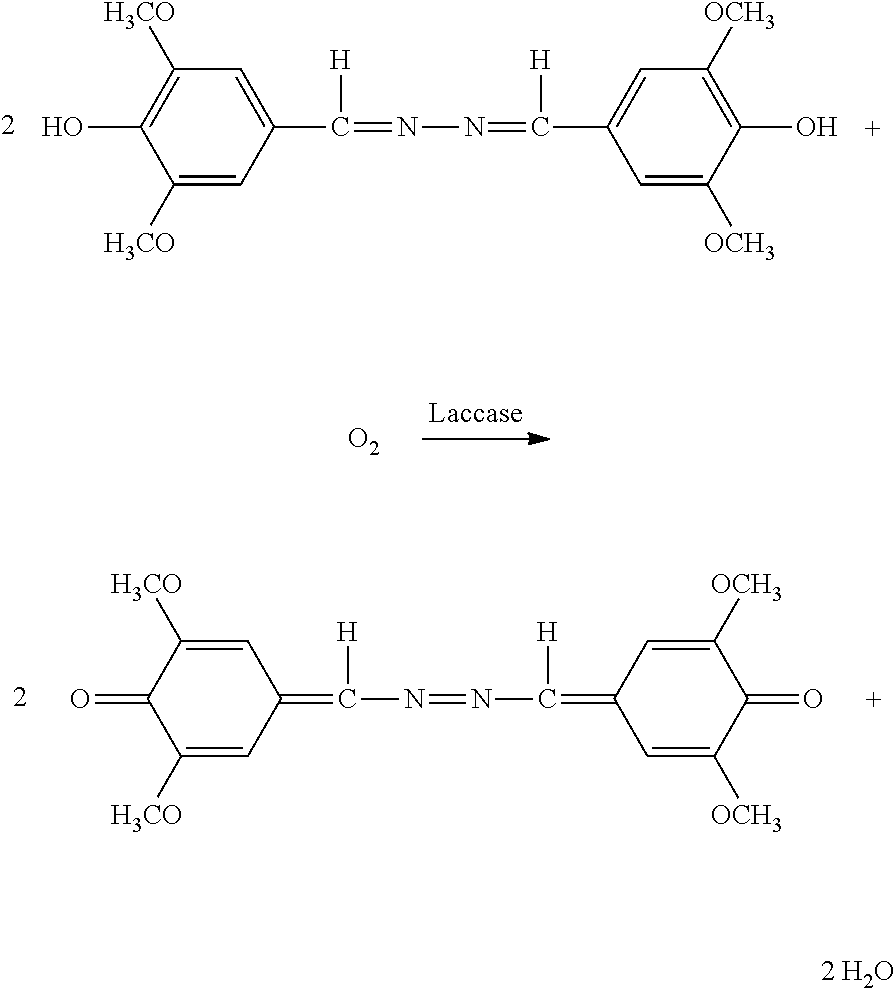
Fusion enzyme and application of fusion enzyme in paper-based biosensor
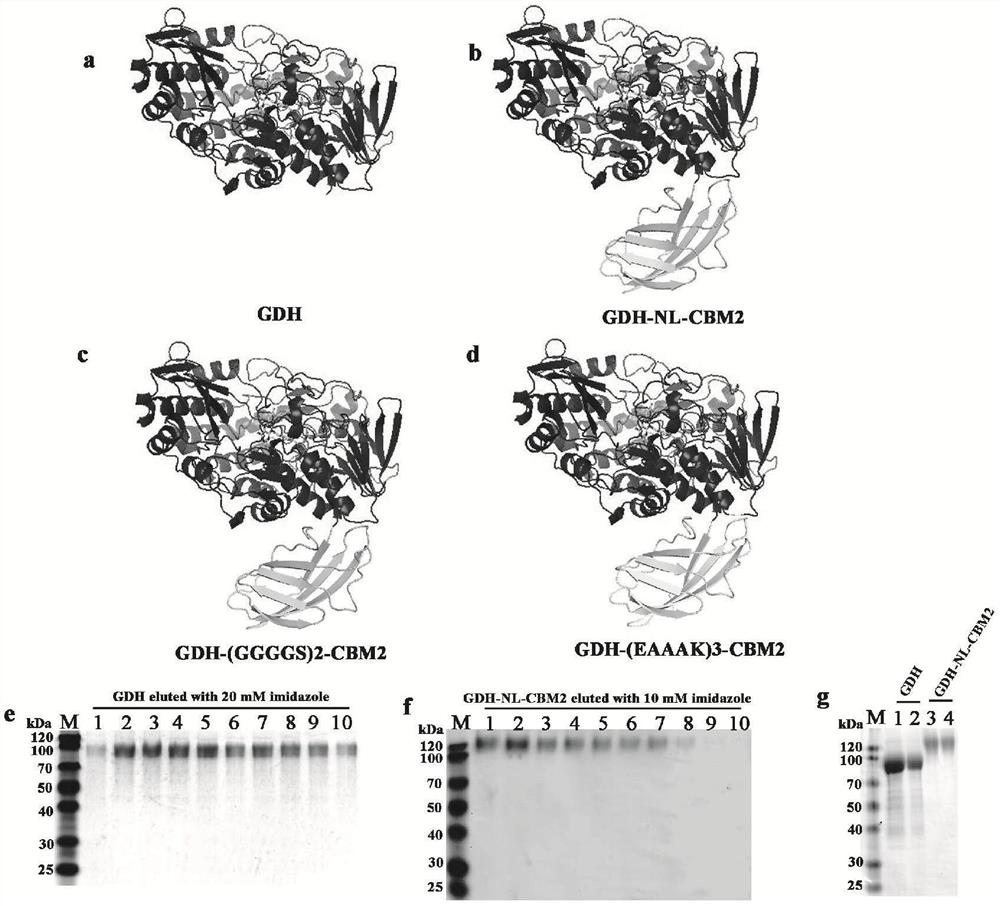
ActiveCN112851821AImprove performanceHigh detection sensitivityPolypeptide with affinity tagOxidoreductasesFlavin adenine dinucleotideGlucose sensors
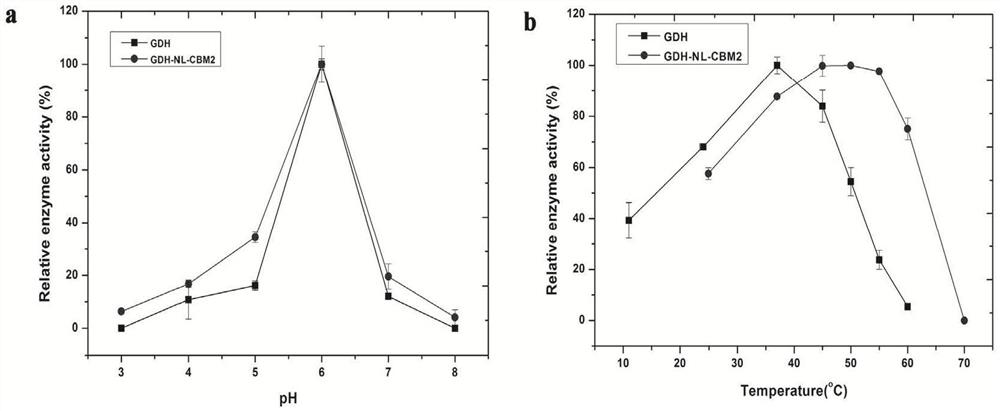
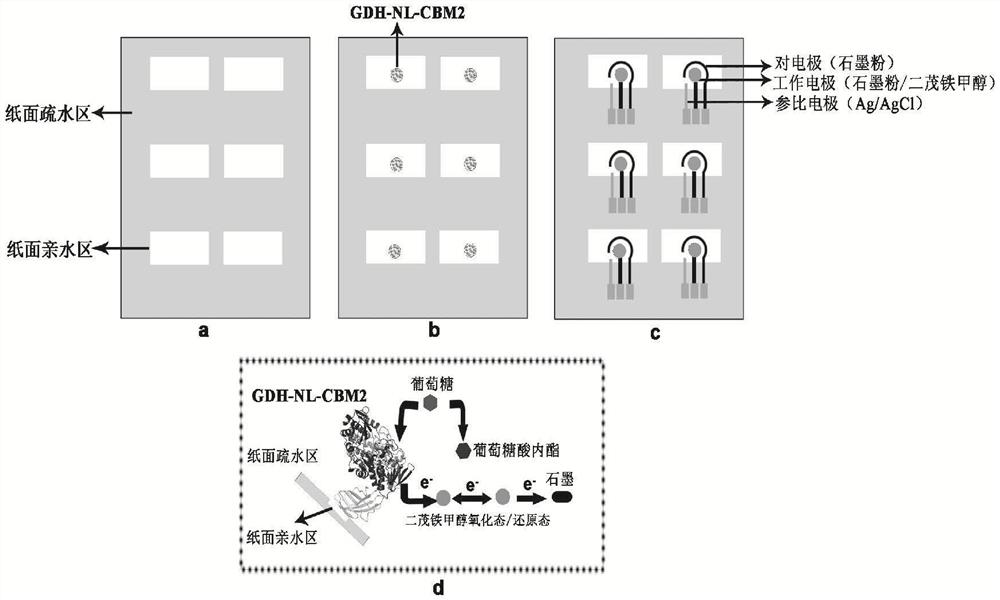
The invention particularly relates to a fusion enzyme and application of the fusion enzyme in a paper-based biosensor. Glucose dehydrogenase (GDH) taking flavin adenine dinucleotide (FAD) as a coenzyme has good stability, is developed into a portable glucose sensor and has a good application prospect. The invention provides a GDH-linker-CBM fusion enzyme constructed by a flavin adenine dinucleotide (FAD) dependent glucose dehydrogenase (GDH) gene and a carbohydrate binding module (CBM) gene, and the GDH-linker-CBM fusion enzyme is prepared into a paper-based sensor through the modification effect of the carbohydrate binding module and a connecting peptide. The enzyme activity, the detection sensitivity and the anti-interference capability of the paper-based sensor are effectively improved, and a good market development prospect is realized.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Polypeptides having alpha-amylase activity and polynucleotides encoding same
ActiveUS20210139875A1Convenient ligationFungiAntibody mimetics/scaffoldsAmylaseCarbohydrate-binding protein
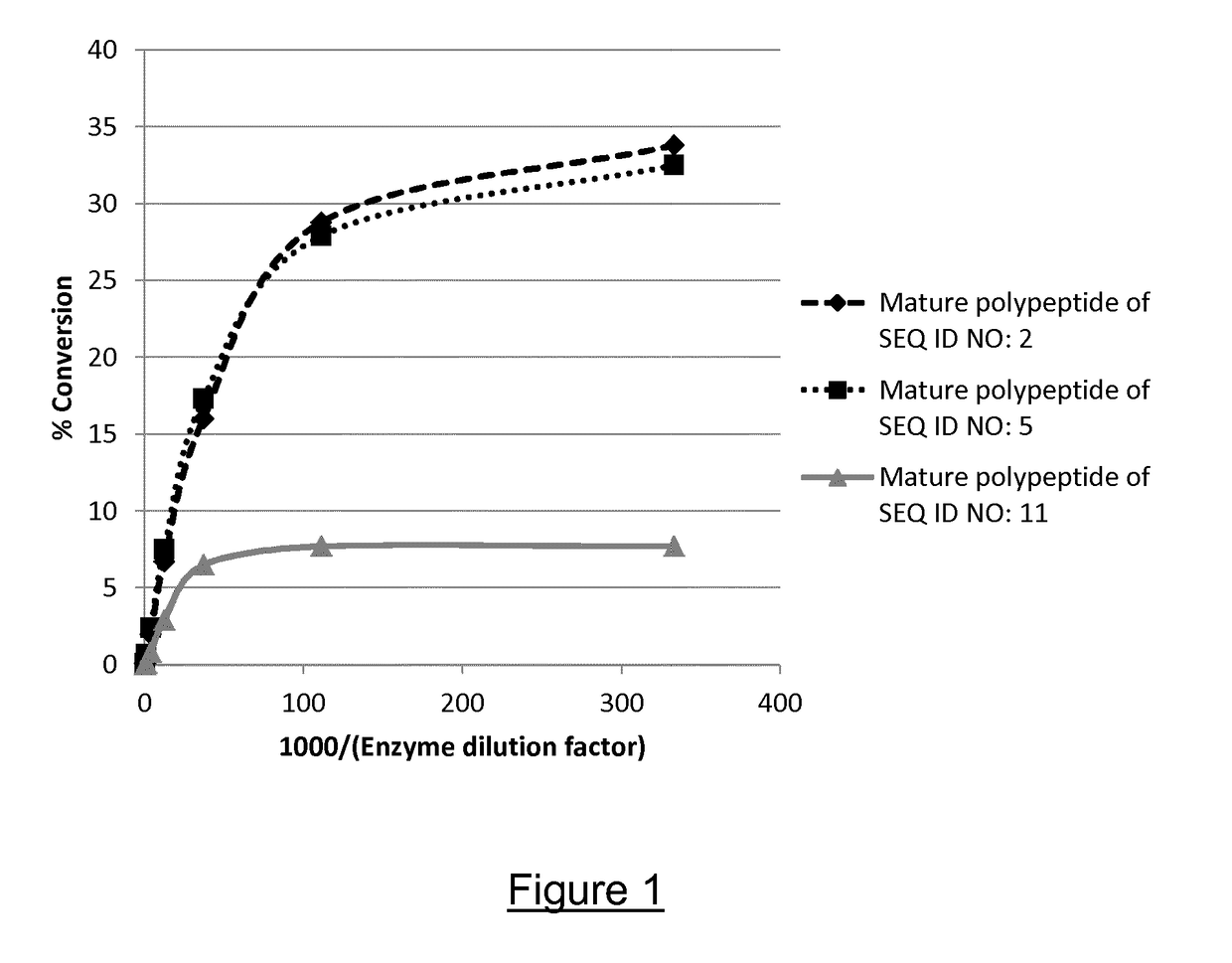
The present invention relates to a hybrid polypeptide having alpha-amylase activity, selected from a first polypeptide sequence comprising a catalytic core, and a second polypeptide sequence comprising a carbohydrate binding module (CBM), wherein (a) the catalytic core is selected from a polypeptide having at least 80%, at least 85%, at least 90%, at least 91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at least 98%, at least 99% or 100% sequence identity to amino acids 20 to 494 of SEQ ID NO: 1 or amino acids 20 to 496 of SEQ ID NO: 1; and (b) the CBM is selected from a polypeptide having at least 75%, at least 80%, at least 85%, at least 90%, at least 91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at least 98%, at least 99% or 100% sequence identity to SEQ ID NO: 4, SEQ ID NO: 5, or SEQ ID NO: 6. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the polynucleotides as well as methods of producing and using the polypeptides, and catalytic domains.
Owner:NOVOZYMES AS
Carbohydrate binding module CBM6B protein capable of specifically recognizing xanthan gum side chains and application
PendingCN112481282APrecise cuttingEfficient targetingBacteriaAntibody mimetics/scaffoldsEscherichia coliSide chain
The invention discloses a carbohydrate binding module CBM6B protein capable of specifically recognizing xanthan gum side chains and application, and belongs to the technical field of biology. According to the invention, a CBM6B gene is cloned from Microbacterium sp. XT11 by using a modern molecular biological means, and then a carbohydrate active enzyme and a fusion expression vector are constructed, and the carbohydrate active enzyme and the fusion expression vector are transformed into Escherichia coli to carry out high-level expression on the fusion enzyme. The CBM6B can improve the affinity and catalytic ability of recombinase and substrate xanthan gum, and lays a foundation for efficiently and accurately cutting xanthan gum and preparing xanthan gum oligosaccharide.
Owner:李宪臻
Polymer probes and methods
ActiveUS10788477B2Quick checkRapid accurate choicePeptide-nucleic acidsBiological material analysisCellulosePolymer science
A polymer detection probe is provided that includes a binding module that specifically binds to at least one polymer and a reporter module that is spectroscopically detectable. The binding module can be a carbohydrate-binding module (CBM). The reporter module can be a fluorescent protein. A complex is provided that includes a probe specifically bound to a pulp or paper product including at least one surface available lignocellulosic polymer. A pulp or paper product is provided that includes at least one surface available lignocellulosic polymer and at least one probe bound thereto. Methods are provided that employ a lignocellulosic probe. A method of detecting a lignocellulosic polymer or other type of polymer is provided. A method of determining the effectiveness of an industrial treatment on pulp or a paper product is also provided. A method of determining a physical property of pulp or a paper product is further provided.
Owner:BUCKMAN LAB INT INC +1
Heat-resistant xylanase and application thereof
ActiveCN105671019AGood activity and stabilityImprove thermal stabilityBacteriaMicroorganism based processesExoxylanase activityXylanase
The invention relates to xylanase and particularly provides a heat-resistant xylanase and an application thereof. The heat-resistant xylanase can be: (a) a nucleic acid for encoding a polypeptide having xylanase activity, wherein a sequence of the protein that is encoded by the nucleic acid is represented as the amino sequence in SEQ ID No.2 (TM1); (b) a nucleic acid for encoding a polypeptide having xylanase activity, wherein the nucleic acid has a sequence having more than 80% homology with the SEQ ID No.2; or (c) a lacked signal sequence or a carbohydrate combination module of the xylanase that encoded by the nucleic acid in the (a) or (b). The xylanase TM1 and TM1-M has huge potential value in the applications of feeds, foods and pulping and papermaking industry.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Polypeptides having cellobiohydrolase activity and polynucleotides encoding same
ActiveUS10519520B2Avoid mistakesExcessive numberFermentationGlycosylasesNucleotideCarbohydrate-binding module
The present invention relates to polypeptides having cellobiohydrolase activity, catalytic domains, carbohydrate binding modules and polynucleotides encoding the polypeptides, catalytic domains or carbohydrate binding modules. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the polynucleotides as well as methods of producing and using the polypeptides, catalytic domains or carbohydrate binding modules.
Owner:NOVOZYMES AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com