Heat-resistant xylanase and application thereof
A technology for xylanase and xylanase activity, which is applied to heat-resistant xylanase and its application fields, can solve the problems of reducing enzyme recycling rate and the like, and achieves excellent properties, strong thermal stability, and thermal stability. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the acquisition of xylanase XynA, TM1, TM1-M and the construction of its prokaryotic expression vector
[0024] The cloning process of the gene and the construction process of its prokaryotic expression vector comprise the following steps:
[0025] 1. Cloning of xylanase gene
[0026] The following primers were designed for the xylanase XynA gene:
[0027] XynA-F:CAAGCAGCCATAACACTCACAT
[0028] XynA-R:TTATTCAATCAACAAATAATCTGCAT
[0029] The following primers were designed for the xylanase TM1 gene:
[0030] TM1-F:CAAGCAGCCATAACACTCACAT
[0031] TM1-R:CGTTGTAGTTGGCGTAGTTGAA
[0032] The following primers were designed for the xylanase TM1-M gene:
[0033] TM1-M-F:CAAACCAGCATAACACTCACATCAAATGCAA
[0034] TM1-M-R:CGTTGTAGTTGGCGTAGTTGAA
[0035] Using the genomic DNA of Thermocellulolyticus saccharolyticum preserved in China General Microbiological Culture Collection (CGMCC) with the preservation number CGMCC1.5183 and the preservation date in March 20...
Embodiment 2
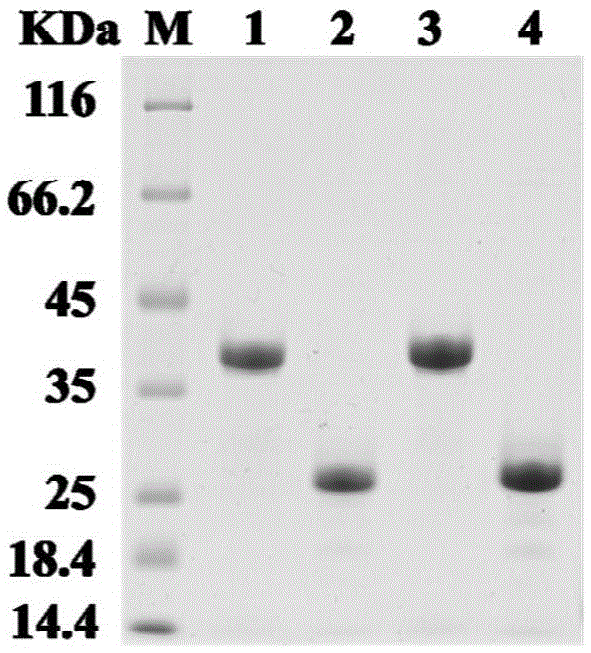
[0092] Embodiment 2, expression and purification of xylanase XynA, TM1, TM1-M
[0093] The prokaryotic expression vectors pEASY-E1-xynA, pEASY-E1-tm1 and pEASY-E1-tm1-m containing xylanase XynA, TM1 and TM1-M genes obtained in Example 1 were transformed into Escherichia coli E.coliBL21 ( DE3), select positive single clones respectively, and shake the positive single clones to OD at 37°C 600 After reaching 0.5, add IPTG with a final concentration of 1mM, induce at 16°C for 16 hours, collect the respective bacterial cells by centrifugation, and add 4ml of phosphate buffered saline (50mM NaH 2 PO 4 , 300mMNaCl, pH8.0), and 0.04ml of 100× protease inhibitor, lysozyme (final concentration 1mg / ml) was added at the same time to fully suspend the bacterial cells and then break the cells by ultrasonic method. The resulting cell disruptions were centrifuged at 4°C and 10,000×g for 20 minutes, and the respective supernatants were filtered through a 0.22 μm filter membrane to become the...
Embodiment 3
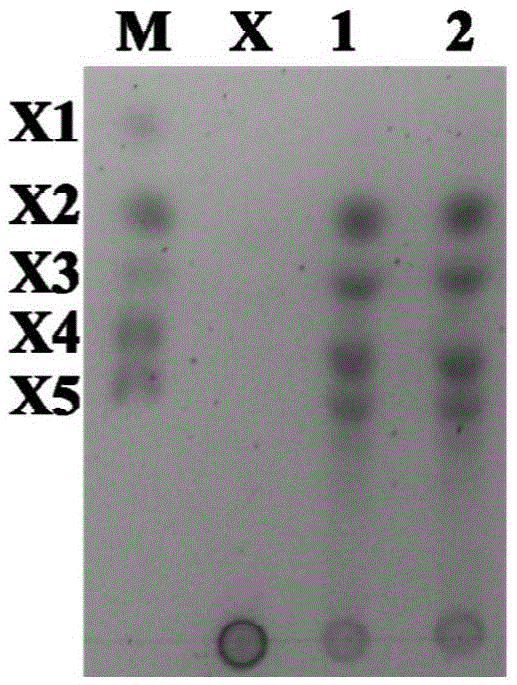
[0095] Embodiment 3, enzymatic characteristic detection
[0096] The xylanases XynA, TM1 and TM1-M obtained by the above method were subjected to the following reactions respectively for identification of enzymatic characteristics:
[0097] 1. Determination of specific enzyme activity of xylanase
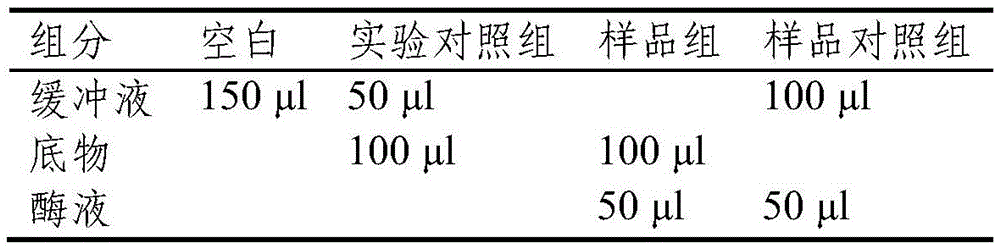
[0098] Utilize the recombinant xylanase of above-mentioned purification, carry out following reaction:
[0099] Take 50 μl each of the 0.8 μg / ml xylanase XynA solution, 0.4 μg / ml truncated protein TM1 solution and 0.4 μg / ml mutant protein TM1-M solution obtained above, and add them to 100 μl 1% Xylan solution, wherein xylan was dissolved in PC buffer (PC buffer is 50mM phosphoric acid, 12mM citric acid, pH6.5), and reacted at 75°C for 10 minutes. The amount of reducing sugar produced after the reaction was determined by 3.5-dinitrosalicylic acid (DNS) method.
[0100] After the above reaction, add 200 μl of DNS solution, boil for 5 minutes, add 650 μl of water after cooling and m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com