Feruloyl esterase as well as mutant N.7-16 and application thereof
A technology of ferulic acid esterase and mutants, which is applied in the field of enzyme engineering, can solve the problems of long time-consuming enzyme production process and low ferulic acid esterase activity, and achieves expansion of preparation methods and acquisition methods, stable properties, and improved enzyme production. live effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
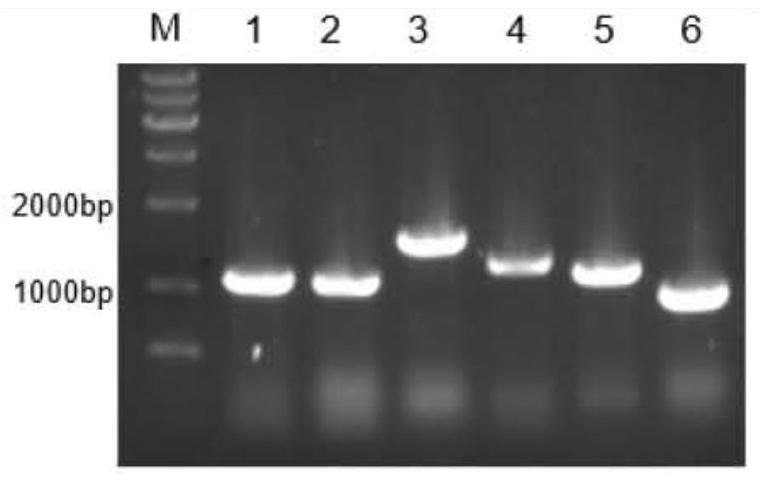
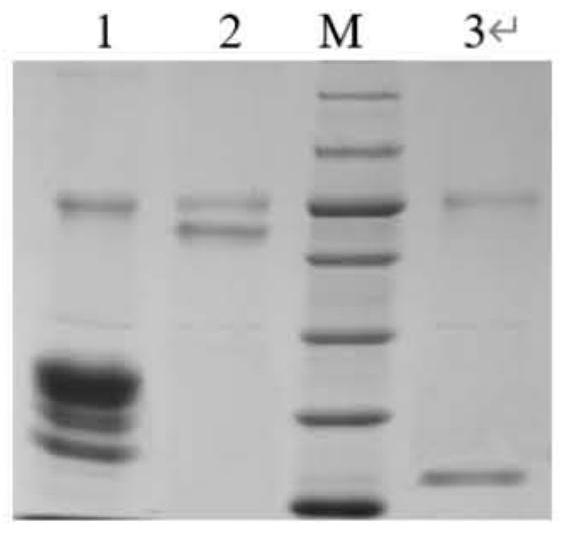
[0036] Example 1: Preparation and purification of ferulic acid esterase
[0037] 1. Preparation of ferulic acid esterase
[0038] 1. PCR amplification reaction
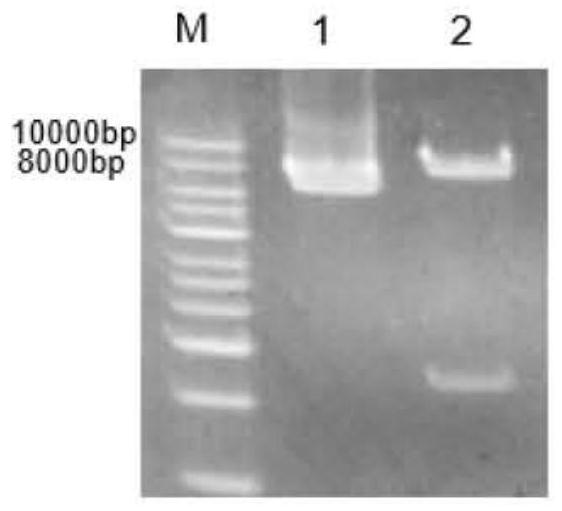
[0039] The amino acid sequences of ferulic acid esterase N.1, ferulic acid esterase N.7 and ferulic acid esterase N.9 (respectively shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 The gene sequence was converted into the gene sequence by Shanghai Sangon Biological Co., Ltd., and the gene sequence was codon-optimized according to the codon preference of the expressing strain Pichia pastoris. Finally, ferulate esters with lengths of 1065bp, 1203bp and 864bp were obtained. Enzyme N.1 gene, ferulic acid esterase N.7 gene, ferulic acid esterase N.9 gene, the nucleotide sequences of which are respectively as SEQ ID No.4, SEQ ID No.5, SEQ ID No.6 shown. Three pairs of amplification primers as shown in Table 1 were designed according to the nucleotide sequence of the ferulic acid esterase gene, and PCR amplification was car...
Embodiment 2
[0116] Example 2: Determination of the enzymatic properties of ferulic acid esterase
[0117] 1. Definition of ferulic acid esterase enzyme activity
[0118] Under certain temperature and pH conditions, the amount of enzyme required to hydrolyze methyl ferulate by ferulic acid esterase to produce 1 μmol of ferulic acid per minute was defined as 1 unit of enzyme activity, expressed as U. The specific enzyme activity of an enzyme refers to the number of units of enzyme activity per mg of ferulic acid esterase under specific conditions.
[0119] 2. Principle of enzyme activity assay
[0120] Ferulic acid esterase hydrolyzes methyl ferulate to form ferulic acid under certain temperature and pH conditions, and calculates the enzymatic hydrolysis of ferulic acid by measuring the change in the absorbance of methyl ferulate in the system before and after the reaction at OD=350 nm. The reduction of methyl ester, and then the activity of ferulic acid esterase.
[0121] 3. Plate scree...
Embodiment 3
[0149] Example 3: Acquisition of ferulic acid esterase mutants
[0150] The nucleotide sequences of the ferulic acid esterase N.1 gene, the ferulic acid esterase N.7 gene, and the ferulic acid esterase N.9 gene obtained in Example 1 were used as templates to compare and analyze the ferulic acid esters. The catalytic domain and substrate-binding domain of the enzyme were site-directed mutagenesis based on their amino acid sequences.
[0151] Ferulic acid esterase N.1-300 was obtained by increasing the number of disulfide bonds of ferulic acid esterase N.1 (nucleotide sequence SEQ ID No. 4), and ferulic acid esterase N.1-300 was composed of The mutant of G300C single point mutation (the amino acid sequence is shown in SEQ ID No. 13, the coding nucleotide sequence is shown in SEQ ID No. 14, the 300th amino acid is changed from glycine to cysteine, the corresponding DNA The sequence changed from GGT to TGT). Change the side chain group of carbohydrate binding module (CBM) of fer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com