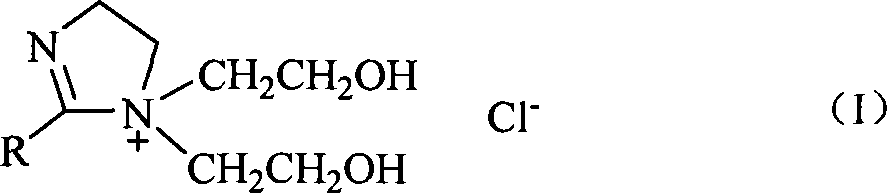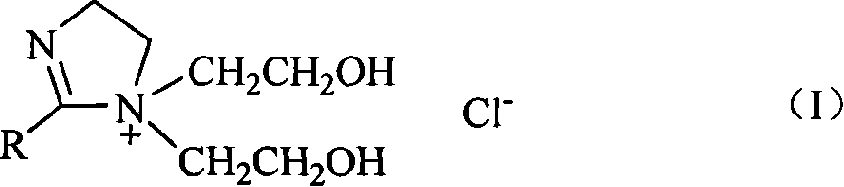Halogen compound oxo reaction method
A carbonylation reaction and halide technology, which is applied in the field of halide carbonylation, can solve the problems of poor water solubility of long-chain alkyl quaternary ammonium salts, low recycling utilization rate, large loss of main catalyst, etc., and achieves good water solubility. , The effect of improved adsorption performance and excellent biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 2-undecyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride is used as a phase transfer catalyst, and benzyl chlorocarbonylation reaction is used to synthesize phenylacetic acid (refer to literature for the synthesis method of phase transfer catalyst: Shi Zhen, Yang Weiguo . Synthesis of Dihydroxyethylimidazoline Cationic Surfactant. Chemical World, 1994, 1: 14-15).
[0029] Catalyst Carbonyl cobalt salt Co(CO) 4 - Synthesis of: Add 0.286g (0.0012mol) CoCl to a 100mL three-neck flask 2 ·6H 2 O, 15mL tetrahydrofuran, pass into CO, add 0.095g (0.0025mol) NaBH in batches 4 , continue to react for 2 hours under the reaction condition of 0-5°C, at this time, the reaction solution is a blue clear solution (cobalt carbonyl salt with better catalytic performance is a green clear solution, without black precipitate).
[0030] Add 1.2 mmol of 2-undecyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride phase transfer catalyst and 10 mL of 30% NaOH aqueous solution to the prepared cobalt...
Embodiment 2
[0032] 2-nonyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride is used as a phase transfer catalyst, and benzyl chlorocarbonylation reaction is used to synthesize phenylacetic acid:
[0033] Add 1.2 mmol of 2-nonyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride phase transfer catalyst and 10 mL of 30% NaOH aqueous solution to the prepared cobalt carbonyl salt, and react at room temperature for 5 min. A solution of 40 mmol benzyl chloride in tetrahydrofuran (30 mL) was added dropwise, and the solution was completed in about 15 minutes, and reacted at 50° C. for 2.5 hours. The organic phase was removed, the aqueous phase was adjusted to acidity with 10% hydrochloric acid solution, and the solution became a pink clear solution. Extract with ether (15mL×3), combine ether extracts, anhydrous MgSO 4 Dry, and recover the phase transfer catalyst solution in the aqueous phase. After filtration and concentration under reduced pressure, phenylacetic acid was precipitated. The product was wh...
Embodiment 3
[0035] 2-nonyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride is used as a phase transfer catalyst, and benzyl chlorocarbonylation reaction is used to synthesize phenylacetic acid:
[0036] Similar to Example 2, except that: the phase transfer catalyst solution recovered in Example 2 and 5 mL of 30% NaOH aqueous solution were added to the prepared cobalt carbonyl salt, and the rest of the reaction conditions were the same. The yield of phenylacetic acid was 52%, and the purity was 99%. The NMR, mass spectrometry, infrared and elemental analysis data were consistent with those reported in the literature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com