Processes for production of organohalogen compound decomposing agents
A technology of organic halogens, manufacturing methods, applied in chemical instruments and methods, catalyst activation/preparation, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] As the raw material iron powder, the average particle size is 100μm and the apparent density is 2.7g / cm 3 , The specific surface area measured by BET method is 0.17m 2 / g, the composition is S: 0.012%, C: 0.26%, O: 1.61%, and the rest is basically reduced iron powder of iron. Add copper sulfate (CuSO 4 ·5H 2 (0) powder, such that the amount of copper in the reduced iron powder is 1% by mass relative to the amount of iron (that is, Cu / Fe=0.01), is charged into a vibration ball mill. In addition, zirconia balls with a diameter of 5 mm were filled into the vibration ball mill in an amount of 50% by volume relative to the internal volume of the ball mill. Next, replace the atmosphere in the ball mill with nitrogen, and operate the ball mill for 4 hours with a vibration number of 1250vpm and an amplitude of 9mm in this state to mechanically mix the reduced iron powder and copper sulfate salt. After the mixing is stopped, the internal powder is taken out into the atmospher...
Embodiment 2~8
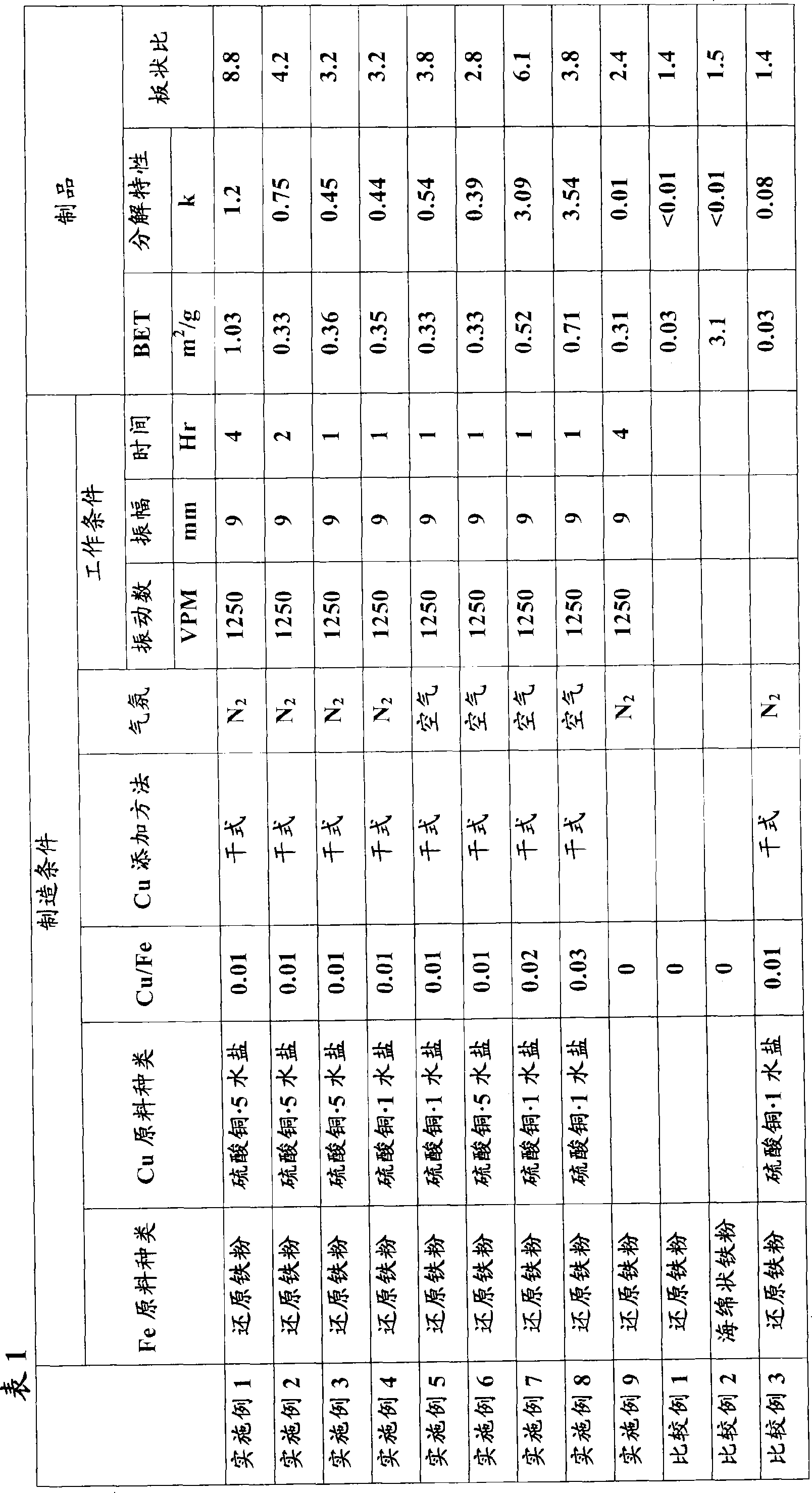
[0043] In addition to the type of copper sulfate used as copper salt powder (that is, copper sulfate with different crystal water content), the mixing ratio of iron powder and copper sulfate (mass ratio of Cu / Fe), the type of replacement gas of the ball mill, the ball mill Example 1 was repeated except that the operating conditions (vibration number, amplitude, and time) were changed to those of each example (Examples 2-8) shown in Table 1. The powders obtained in each example were evaluated in the same manner as in Example 1, and the results are shown in Table 1 together.
[0044]
[0045] From the results in Table 1, the following can be known.
[0046] Examples 1 to 3 were produced under the same conditions except for changing the operating time of the ball mill. The longer the operating time, the larger the plate shape ratio and the larger the k value.
[0047] Examples 5, 7, and 8 were produced under the same conditions except that the mixing ratio of Cu / Fe was change...
Embodiment 3
[0048] Examples 3 and 5 were produced under basically the same conditions except for using copper sulfate with different amounts of water of crystallization. Compared with Example 3 using copper sulfate pentahydrate, the example using copper sulfate monohydrate The k value of 5 is higher, and it can be seen that the decomposition performance is higher when copper salt powder with less crystal water is used.
[0049] Examples 4 and 5 and Examples 3 and 6 were produced under the same conditions except that the ambient atmosphere in the ball mill was changed to nitrogen and air, and it can be seen that the k value is larger in the nitrogen atmosphere.
[0050] It should be noted that for the powders obtained in Examples 1 to 8 above, in the decomposition performance test, when the test was carried out in the same manner using 50 ml of unaerated pure water instead of kaolin, at the time of the initial measurement after 1 hour, cis The gas concentrations C of -1,2-DCE were all belo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com