Biinomenine derivative connected with C-C bond, preparation method and application thereof
A bis-sinomenine, biological preparation technology, applied in the direction of medical preparations containing active ingredients, drug combinations, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
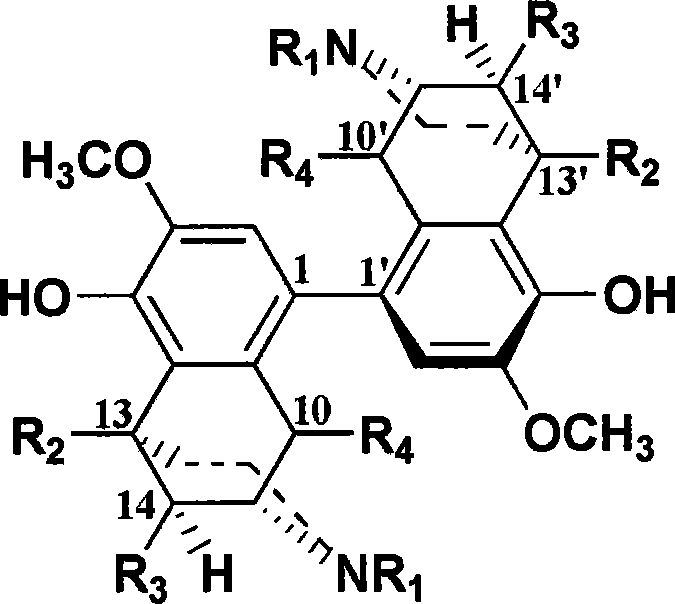
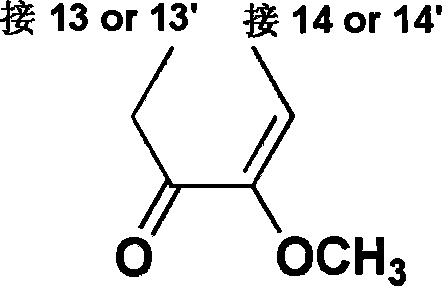
[0047] The stereoselective biosynthesis of carbon-carbon-linked double sinomenine, the synthetic route is:
[0048]
[0049] The strain Antrodiella semisupina was cultured on a slant medium at 25°C for 7 days, then inoculated into sterilized 2L transformation medium, cultured on a shaker for 4 days (25°C, 150r / min), and added through a 0.1μm microporous filter membrane Sinomenine hydrochloride was added at a concentration of 250μg / ml, and the culture was continued for 4 days under the same conditions. The transformed liquid was filtered, and the hyphae was rinsed with a small amount of distilled water with pH 6.0. After combining, a total of 2100ml of filtrate was obtained. The pH value was adjusted to 8-9 with ammonia water, and extracted with dichloromethane several times. The dichloromethane extracts were combined and anhydrous sulfuric acid. Dry with sodium, filter, and spin to evaporate the solvent to obtain 450 mg of solid powder. The transformed product was separated by a...
Embodiment 2
[0053] The stereoselective biosynthesis of carbon-carbon-linked bis-deazamethylsinomenine, the synthetic route is:
[0054]
[0055] The method is the same as in Example 1, but the substrate is N-norsinomenine, and 100 mg of deazamethylbissinomenine is obtained, and the yield is 20%.
[0056] Norbissinomenine A(3): positive ion ESI-MS m / z 609[M+H] + .
[0057] Hydrogen spectrum (solvent Chloroform-d): 6.30 (1H, s, 2-H), 3.75 (3H, s, 3-OCH3), 2.51 (1H, d, J = 15.6 Hz, 5-H), 4.37 ( 1H, d, J = 15.6 Hz, 5-H), 3.49 (3H, s, 7-OCH3), 5.38 (1H, d, J = 2.4 Hz, 8-H), 3.36 (brt, J = 4.0 Hz, 9-H), 2.75 (1H, dd, J=18.4, 5.6Hz, 10-H), 2.19 (1H, brd, J=18.4Hz, 10-H), 2.90 (1H, brs, 14-H), 2.02 (1H, brd, J = 12.2 Hz, 15-H), 1.72 (1H, td, J = 13.0, 3.9 Hz, 15-H), 2.85 (1H, dd, J = 13.0, 3.9 Hz, 16-H) ), 2.60 (1H, td, J=13.0, 3.9 Hz, 16-H). The other half from 1'to 17' is the same as 1 to 17.
[0058] Carbon spectrum (solvent Chloroform-d): 131.0(C-1), 110.4(C-2), 145.1(C-3), 56.0(C-3-OCH3), 143....
Embodiment 3
[0061] The chemical synthesis of carbon-carbon-linked double sinomenine, the synthetic route is:
[0062]
[0063] Method A: Add 200 mg (0.61 mmol) of sinomenine to the reaction flask, dissolve it in 20 ml of methanol, add 1 g (11.5 mmol) of manganese dioxide, and stir at room temperature to react for 48 hours. After centrifugal separation, the supernatant was taken and concentrated under reduced pressure to obtain a brown oil. White compounds 1 (about 60%) and 6 (about 40%) were separated by silica gel column chromatography (chloroform-methanol 15:1V / V).
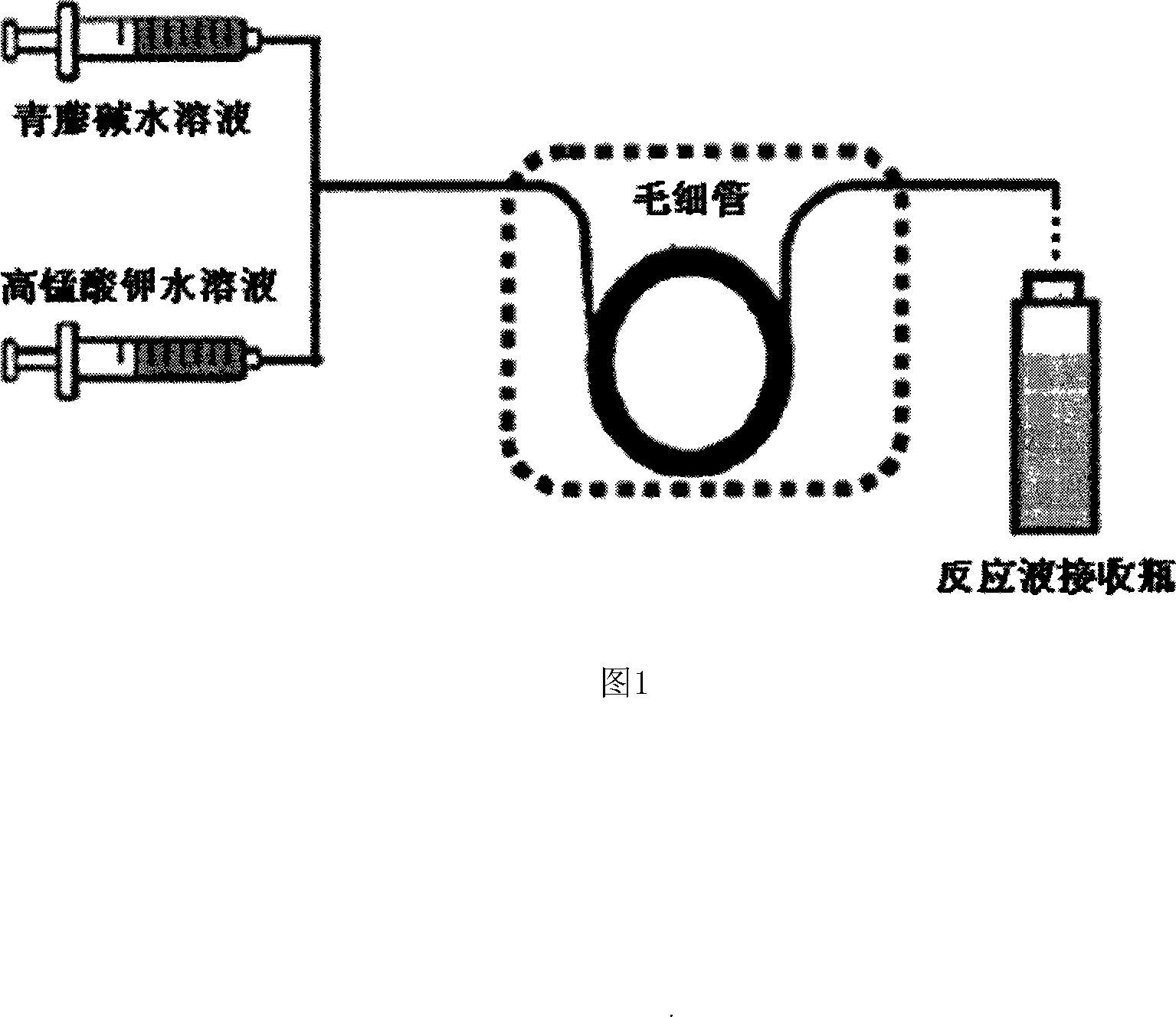
[0064] Method B: Add 200 mg (0.61 mmol) of sinomenine hydrochloride to the reaction flask, dissolve it in 20 ml of water, add 10 ml of 50 mM potassium permanganate solution, stir at room temperature, and check the progress of the reaction by TLC. When the reaction is terminated, ammonia water is added to adjust the pH to 8.0-9.0, and the mixture is extracted with 100 ml of dichloromethane. The organic phase is dried with anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com