Histone deacetylase inhibitor and its preparation method and use
A compound and unsaturated technology, applied in drug combination, organic chemistry, pharmaceutical formulation, etc., can solve the problem of non-selectivity of HDACs and achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
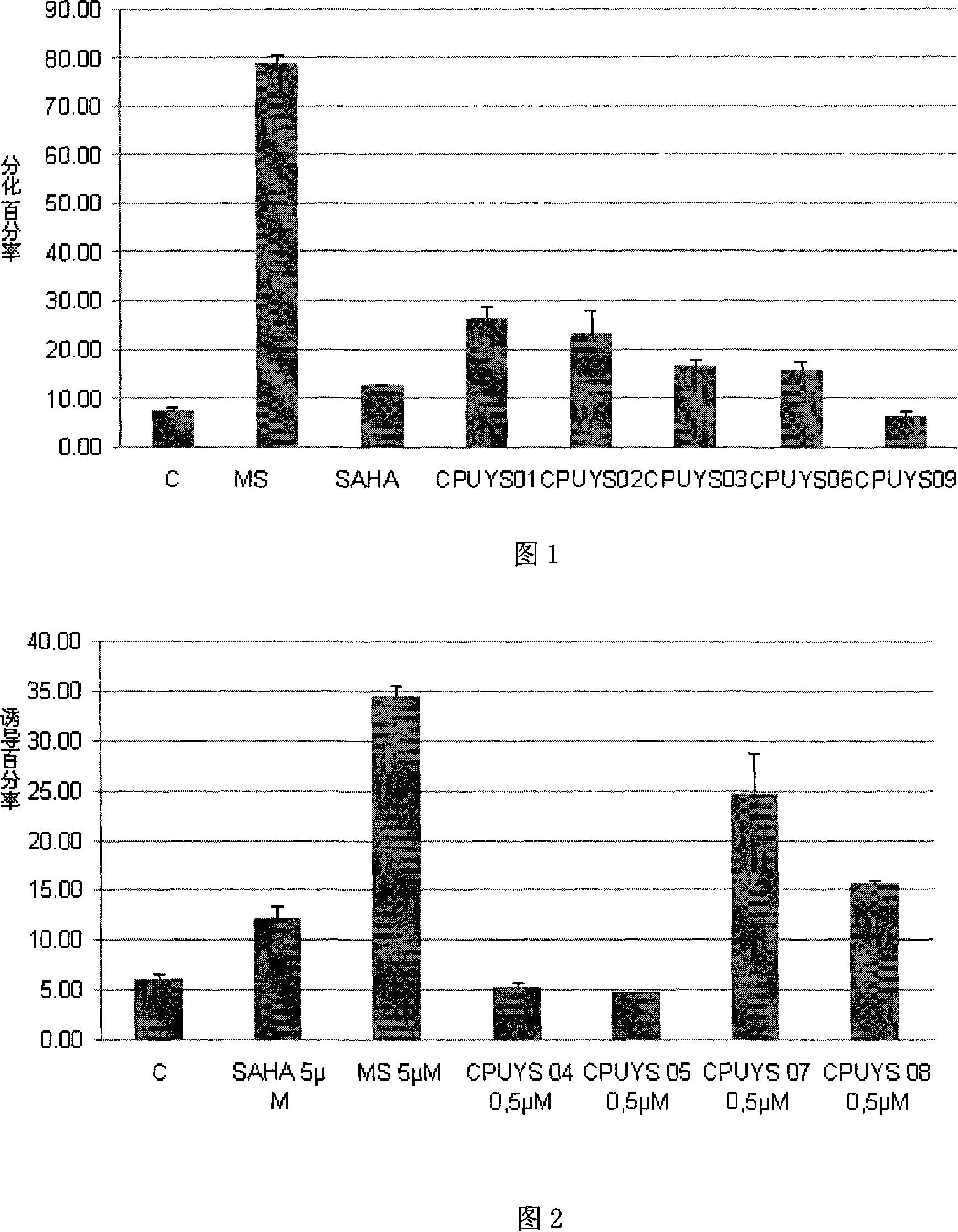
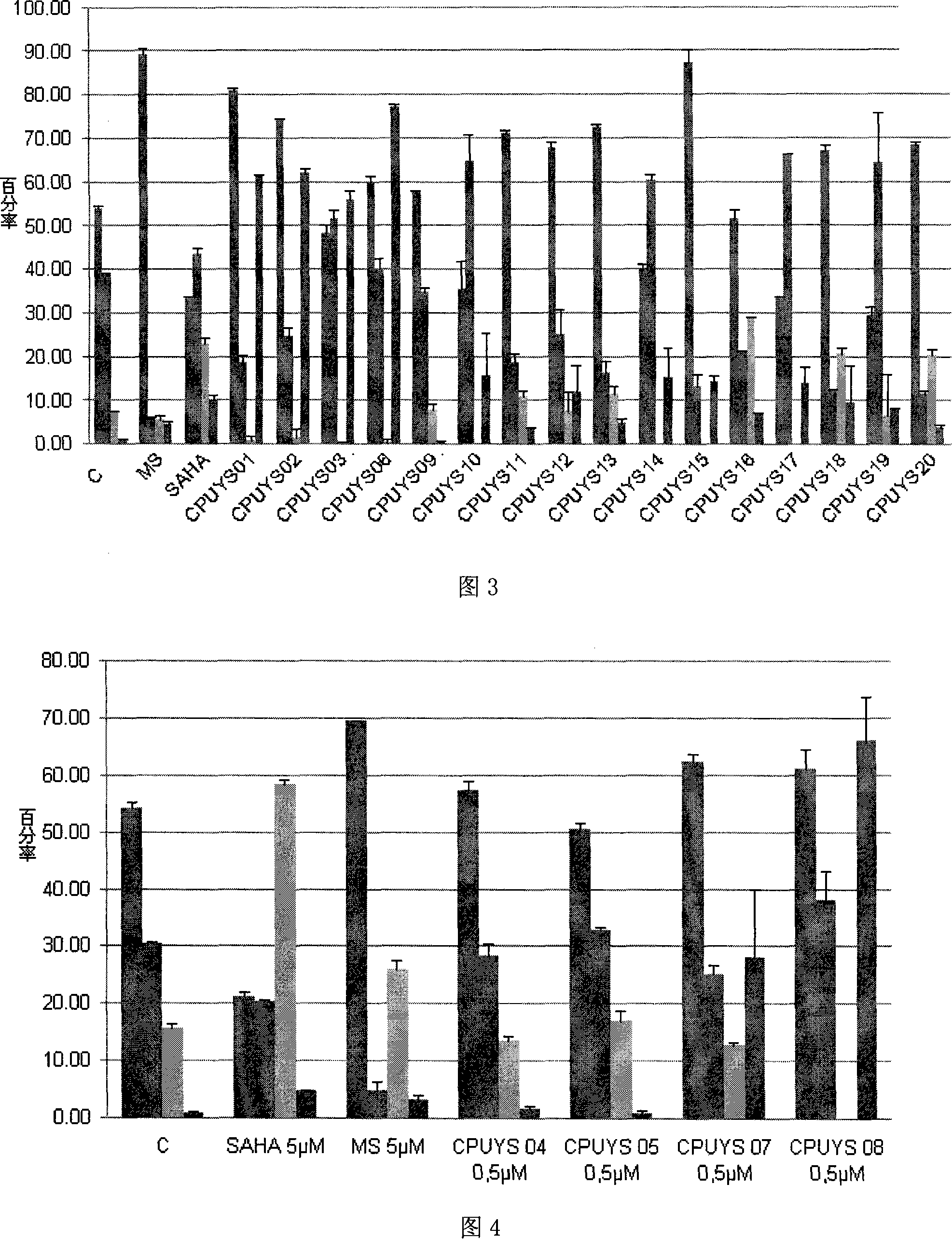
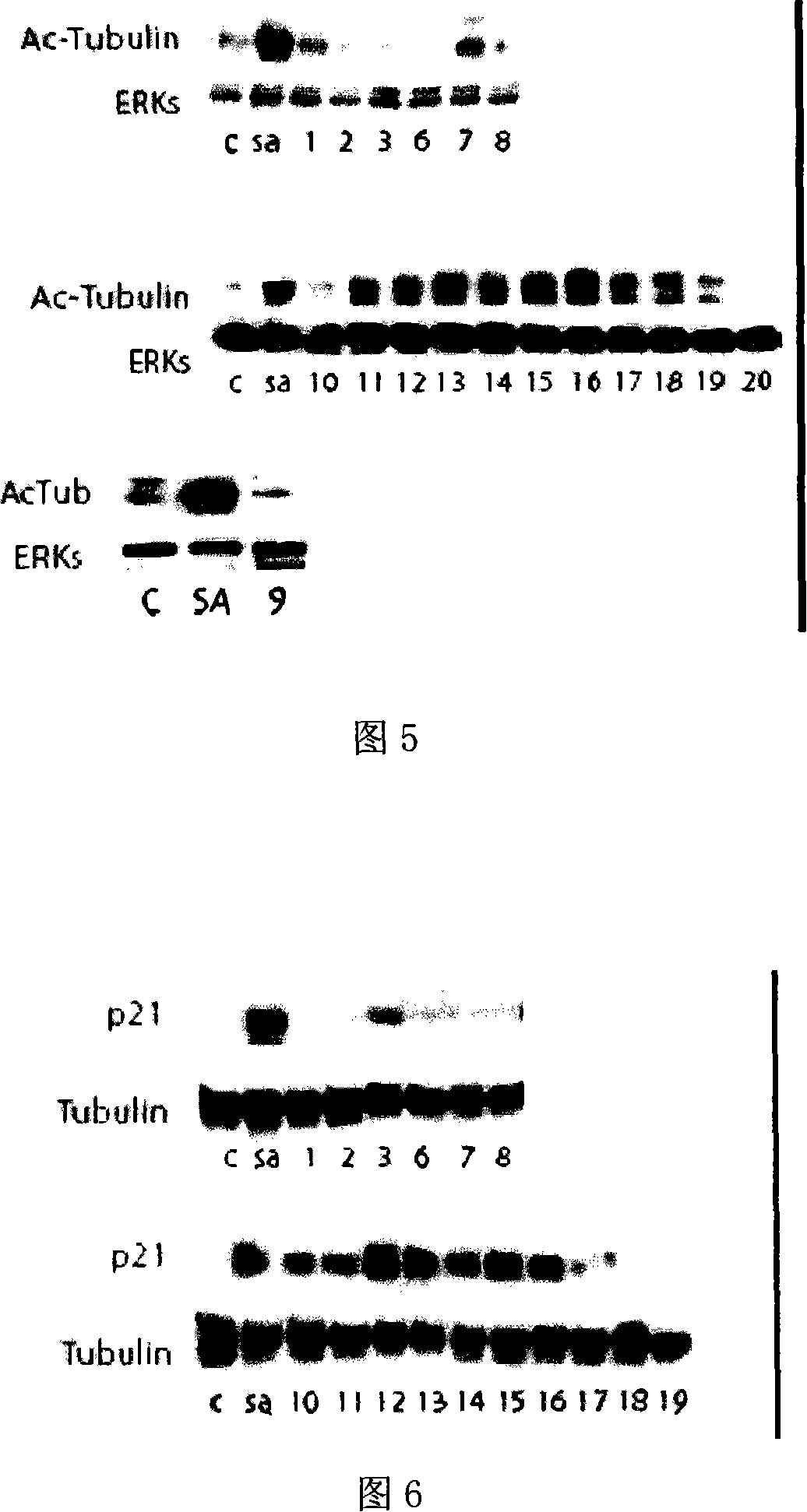
Image
Examples
Embodiment 1
[0098] Preparation of 5-chloromethyl, ethyl 2-furoate
[0099]
[0100] Add 14.0 grams (0.1 mol) of ethyl 2-furoate into a three-necked flask, then add 100 milliliters of anhydrous chloroform to dissolve to obtain a clear solution, then add 3.0 grams (0.1 mol) of paraformaldehyde, 27.2 grams (0.2 mol) of chlorine zinc oxide. Pass dry hydrochloric acid gas into the reaction solution, heat to 35°C, stir the reaction until the reaction is complete, then stop the reaction. After the reaction, the reaction solution was divided into two layers. The reaction solution was poured into 50 ml of ice water, separated, and the chloroform layer was taken and dried over anhydrous sodium sulfate. Concentrated and distilled under reduced pressure to obtain the product, 12.7 g of oily substance, yield 65%.
Embodiment 2
[0102] (E)--5-styryl, the preparation of ethyl 2-furanoate
[0103]
[0104] Add 4.50 g (0.01 mol) of 5-chloromethyl, ethyl 2-furoate triphenylphosphine salt, 1.06 g (0.01 mol) of benzaldehyde, 5.6 ml (0.04 mol) of triethylamine and 50 ml Dichloromethane was heated to reflux for 4 hours. After the reaction solution was cooled, the reaction solution was poured into 100 milliliters of petroleum ether, a large amount of white precipitates were precipitated, suction filtered, the filter cake was washed with petroleum ether, the filtrates were combined, concentrated, and column chromatography gave 1.34 grams (52.6%) of the product . 1 H NMR (300MHz, DMSO-d 6 ): δppm: IR(KBr)cm -1 :
Embodiment 3
[0106] (E)--5-phenethyl, the preparation of ethyl 2-furanoate
[0107]
[0108] Add (E)--5-styryl, 1.15 g (0.005 mol) of ethyl 2-furancarboxylate into the reaction flask, add methanol to dissolve, add a catalytic amount of palladium carbon (10%), and catalytically hydrogenate at room temperature (5 bar) . The reaction stopped after 5 hours. The remaining palladium carbon was removed by suction filtration, the filtrate was concentrated and recrystallized to obtain 1.06 g of the product. Yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com