Regrouped anticancer peptide, producing method and application of the same
A technology of anti-cancer peptides and recombinant plasmids, which is applied in the direction of peptides, anti-tumor drugs, drug combinations, etc., can solve the problems of restricting the application of anti-cancer drugs, difficulty in synthesis, and low content of peptides, so as to facilitate artificial synthesis and industrialization The effects of normalization, inhibition of invasion and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Recombination, identification, purification and collection of anticancer peptides of the present invention.
[0020] Using the IMPACT-TWIN system of NEB (New England Biolabs), the preparation of recombinant cyclic small peptides was realized through chitin bead affinity chromatography and intein self-splicing function.
[0021] 1. Construction of recombinant plasmid pTWIN2-RGD
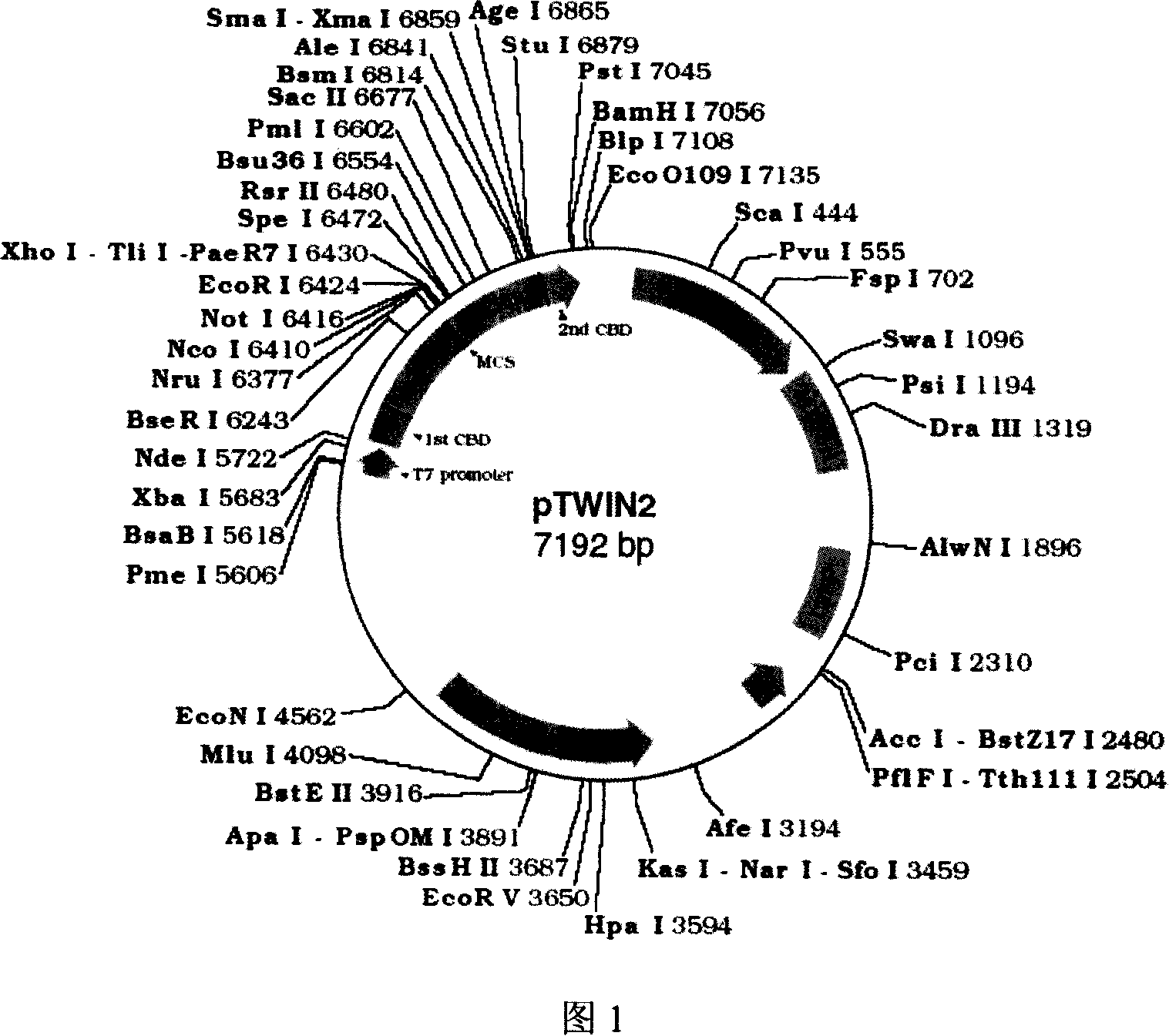
[0022] The vector map of pTWIN2 is shown in Figure 1.
[0023] The partial sequence of the pTWIN2 vector is as follows:
[0024] Polylinker Region: pTW IN2
[0025] 5’...AC TGG GAC TCC ATC GTT TCT ATT ACG GAG ACT GGA GTC GAA GAG GTT TTT
[0026] Ssp DnaB Intein Forward Primer→
[0027] ←Intein
[0028] ...Ssp DnaB Intein... Val Ala Asn Asp Ile Ile Val His Asn
[0029] GAT TTG ACT GTG CCA GGA CCA CAT AAC TTT G TC GCG A AT GAC ATC ATT GTA CAC AAC
[0030] ...
Embodiment 2
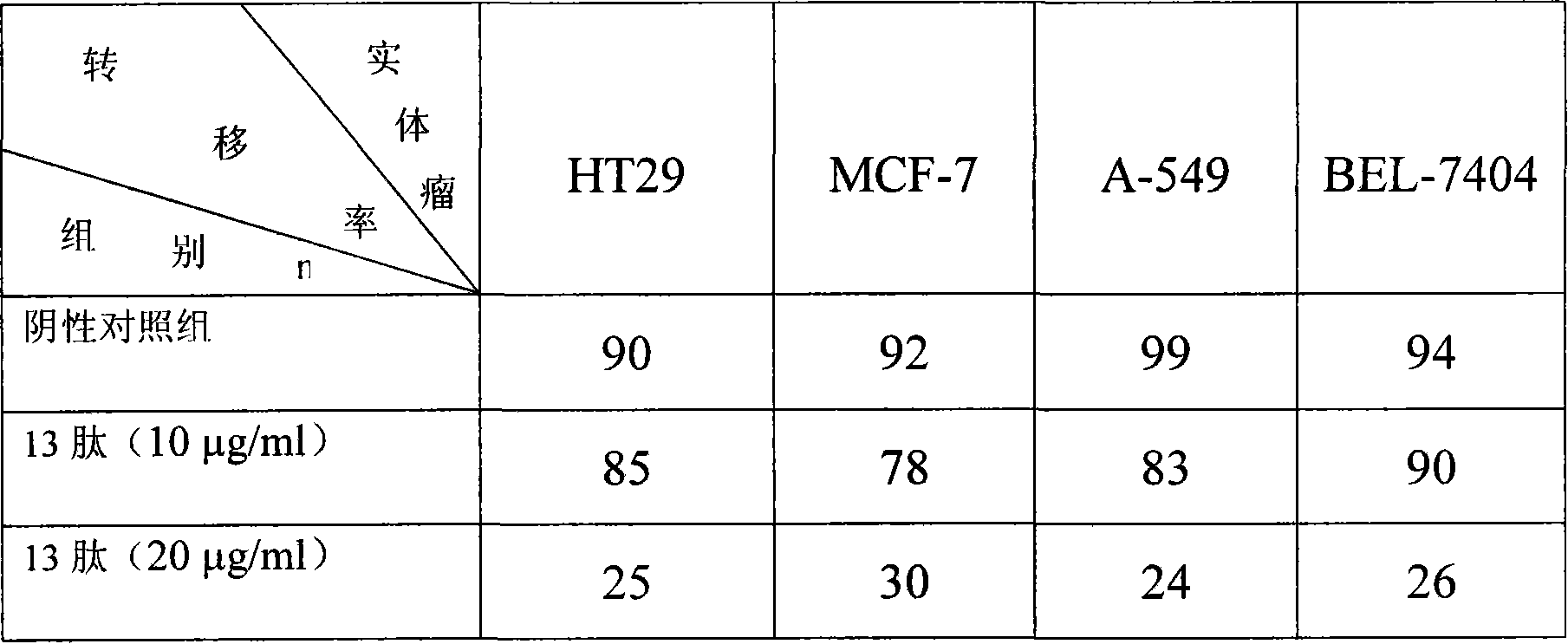
[0088] Example 2 The inhibitory effect of the anticancer peptide of the present invention (hereinafter referred to as recombinant 13 peptide) on tumor cell migration.
[0089] Cells were seeded on glass slides, and after the cells grew to 100% confluence, the slides were taken out and scratched on the slides with a sterile tip. After the scraped cells were washed with PBS, the slides were placed in a place containing 13 peptide (10 , 20μg / ml) culture solution, continue to incubate for 24 hours, take out, fix and HE staining. Observe the cell wound healing under a low power microscope. Take 3 fields of view randomly, count the number of migrating cells, and express the cell density Migration activity. Blank medium was used as negative control. See Table 1 for detailed results.
[0090] Table 1: Inhibitory effect of recombinant 13 peptide on cell migration (take 20 μg / ml as an example)
[0091] cell type
negative control group
13 peptide treatment groups
...
Embodiment 3
[0094] Example 3 Inhibitory effect of recombinant 13 peptide on tumor cell invasion
[0095]Cells were seeded on a microporous filter membrane with a diameter of 5 microns, and the filter membrane was placed between the upper and lower layers of a Boyden chamber (inoculated cells face up), and the culture solution containing 13 peptides (10, 20 μg / ml) was added to the upper chamber (Negative control as above), add medium containing 10% calf serum to the lower chamber, incubate at 37°C for 6 hours, scrape off the cells on the inoculated surface with a cotton swab after taking it out, then fix the membrane, and take 3 fields of view under the microscope , counting the number of cells that penetrated to the back of the membrane, which indicates the invasion ability of the cells. See Table 2 for detailed results.
[0096] Table 2:
[0097] cell type
negative control group
13 peptide treatment groups
Vascular smooth muscle cells
MCF-7
OVCAR
HT2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com