Method for improving volume of production of rifamycin
A high-yield, high-quality technology, applied in the field of increasing the yield of rifamycin, can solve problems such as low success rate, unstable high-yielding strains, and prone to reverse mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of DNA probes required for cloning two-component systems
[0048] Among the currently known response regulator proteins, OmpR (Escherichia coli DNA binding protein) is a well-studied bacterial regulatory protein (Parkinson JS and Kofoid EC. Communication modules inbacterial signaling proteins. Annu Rev Genet.1992; 26: 71-112 ), the three-dimensional structure obtained by X-ray crystallography shows that its C-terminus has a very typical α-helix-loop-α-helix DNA binding region. OmpR proteins can enhance the transcription function of RNA polymerase by binding to the promoter region of the gene, so as to regulate the transcription of the corresponding gene. According to the conserved amino acid sequence of OmpR family proteins (GLEVGADD near the N-terminal, TVRGVGY near the C-terminal) (Wray LVJr, Fisher SH. The Streptomyces coelicolor glnR gene encodes a protein similar to other bacterial response regulators. Gene.1993Aug 16; 130 (1):145-50), desig...
Embodiment 2
[0049]Example 2: Screening of amrE and amkE genes from the A. mediterranei U-32 genome library
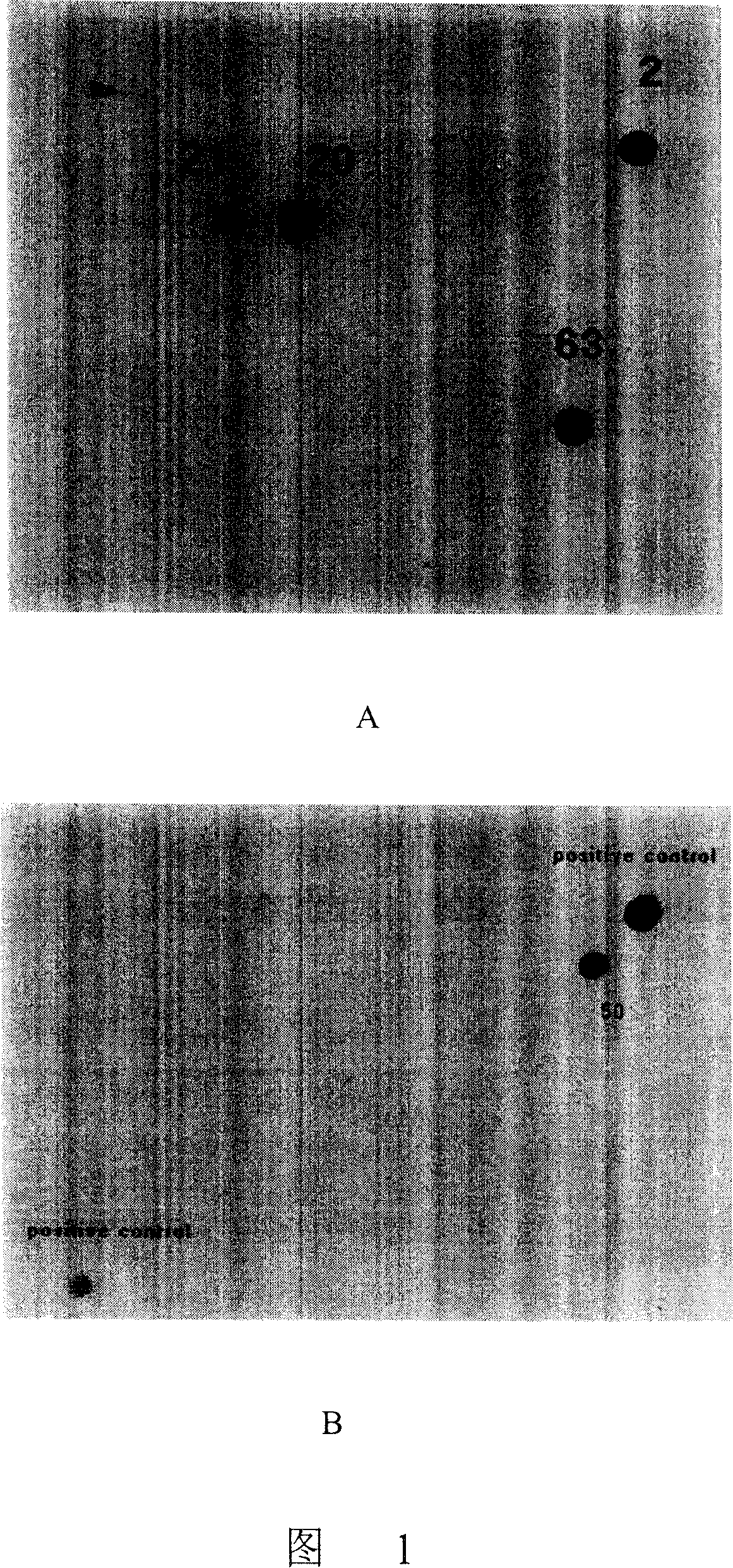
[0050] Due to the existence of OmpR protein gene in Escherichia coli DH5α (Gibco-BRL), it is difficult to screen the correct positive clones by using bacterial colony in situ hybridization, so the dot DNA hybridization method was used for screening. 46-50 colonies were divided into one group to extract mixed plasmids, and a total of 80 groups of mixed plasmids (equivalent to 3800 recombinant plasmids) were extracted. Using a 96-well dot blot injector, spot the denatured mixed plasmids on a nylon membrane, and then hybridize with the DNA probe prepared in Example 1. Select stringent conditions for washing the membrane, and as a result, 4 positive hybridization points were obtained on the membrane, numbered 2, 20, 21, and 63 (see Figure 1A), and the plasmids of 50 colonies corresponding to No. 63 were extracted for the second round After hybridization screening, the recombinant clon...
Embodiment 3
[0051] Example 3: Sequencing and Analysis of Amycobacterium mediterranei U-32amrE and amkE Gene Sequences
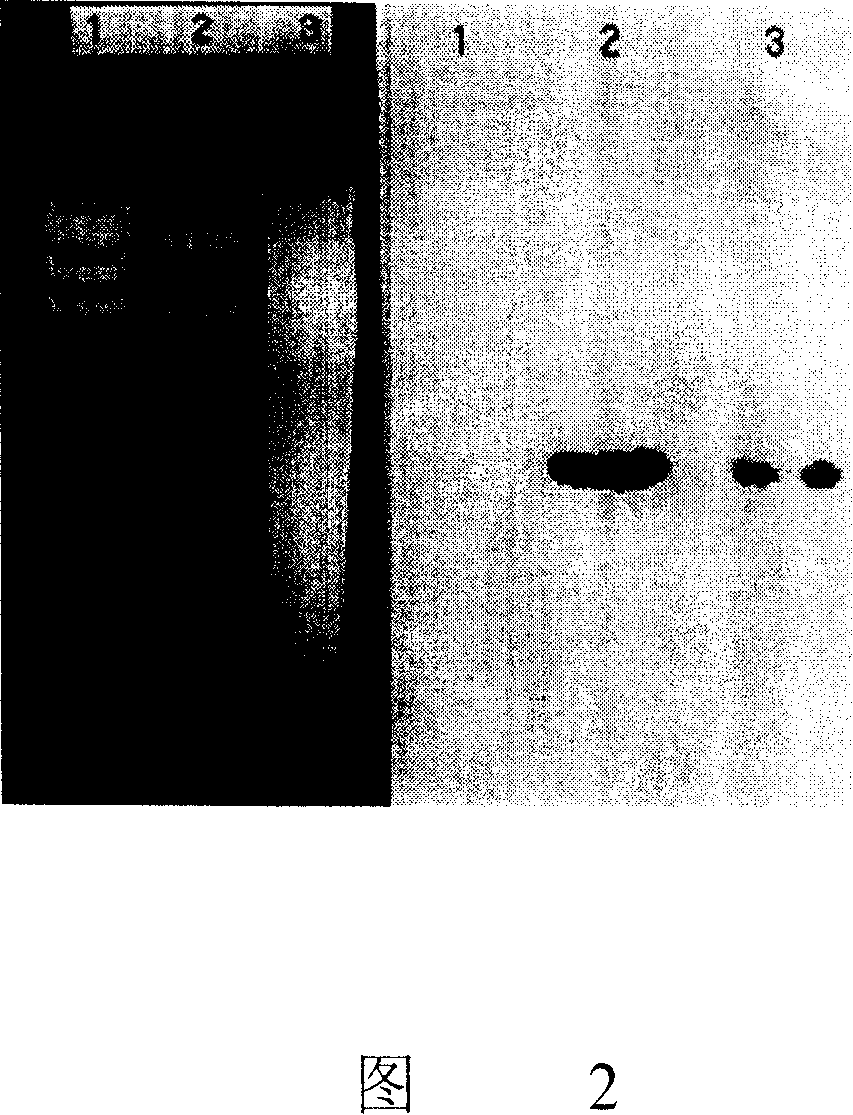
[0052] Plasmid pLR6350 was digested with BglII, StuI, SacI, PstI, XhoI enzymes, electrophoresis, Southern transfer, and hybridized with the DNA probe prepared in Example 1. The results showed that BglII, StuI, SacI, PstI, XhoI enzymes were digested respectively Single hybridization bands of 2.5 kb, 12 kb, 2.0 kb, 1.2 kb and 4.6 kb were generated. The 2.5 kb BglII restriction fragment and the 2.0 kb PstI restriction fragment were respectively cloned into the BamHI and PstI sites of pBlueScriptIIKS vector (Stratagene) to obtain plasmids pKS2.5B and pKS2.0P. The plasmids pKS2.5B and pKS2.0P were sequenced to obtain a DNA sequence of 3238bp (see FIG. 3 ).
[0053] Sequence analysis showed that there were two closely linked complete reading frames (ORFs) on the sequenced 3.2kb DNA fragment. ORF1 starts from ATG at position 456 and ends at TGA at position 1139, with a full l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com