Therapeutic agent for disease with apoptotic degeneration in eye tissue cell containing PEDF and FGF2
A technique for tissue cells and diseases, applied in the field of therapeutic drugs containing PEDF and FGF2 for diseases accompanied by apoptosis and degeneration of eye tissue cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1V
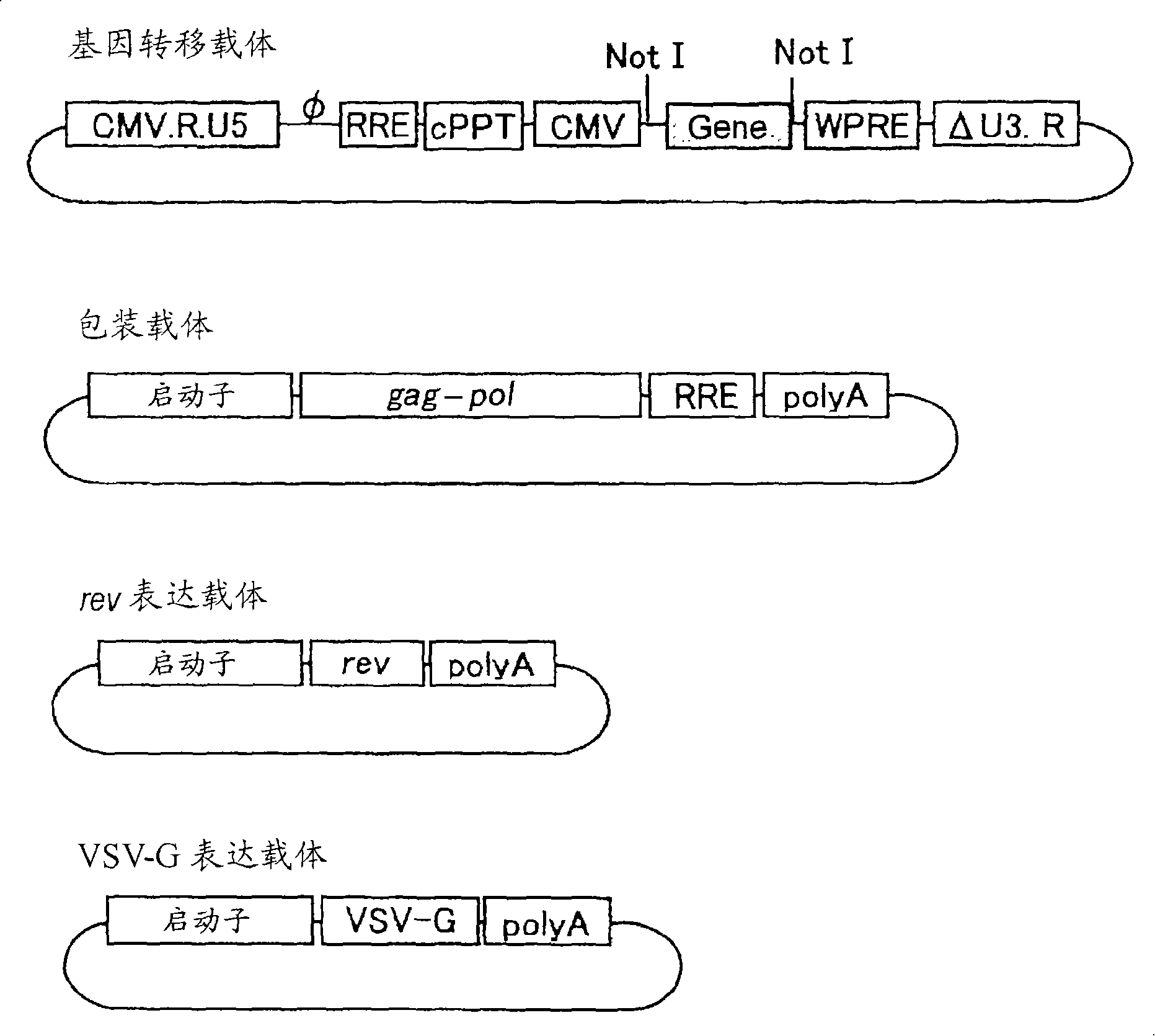
[0098] Example 1 Construction of VSV-G pseudotyped SIV vector
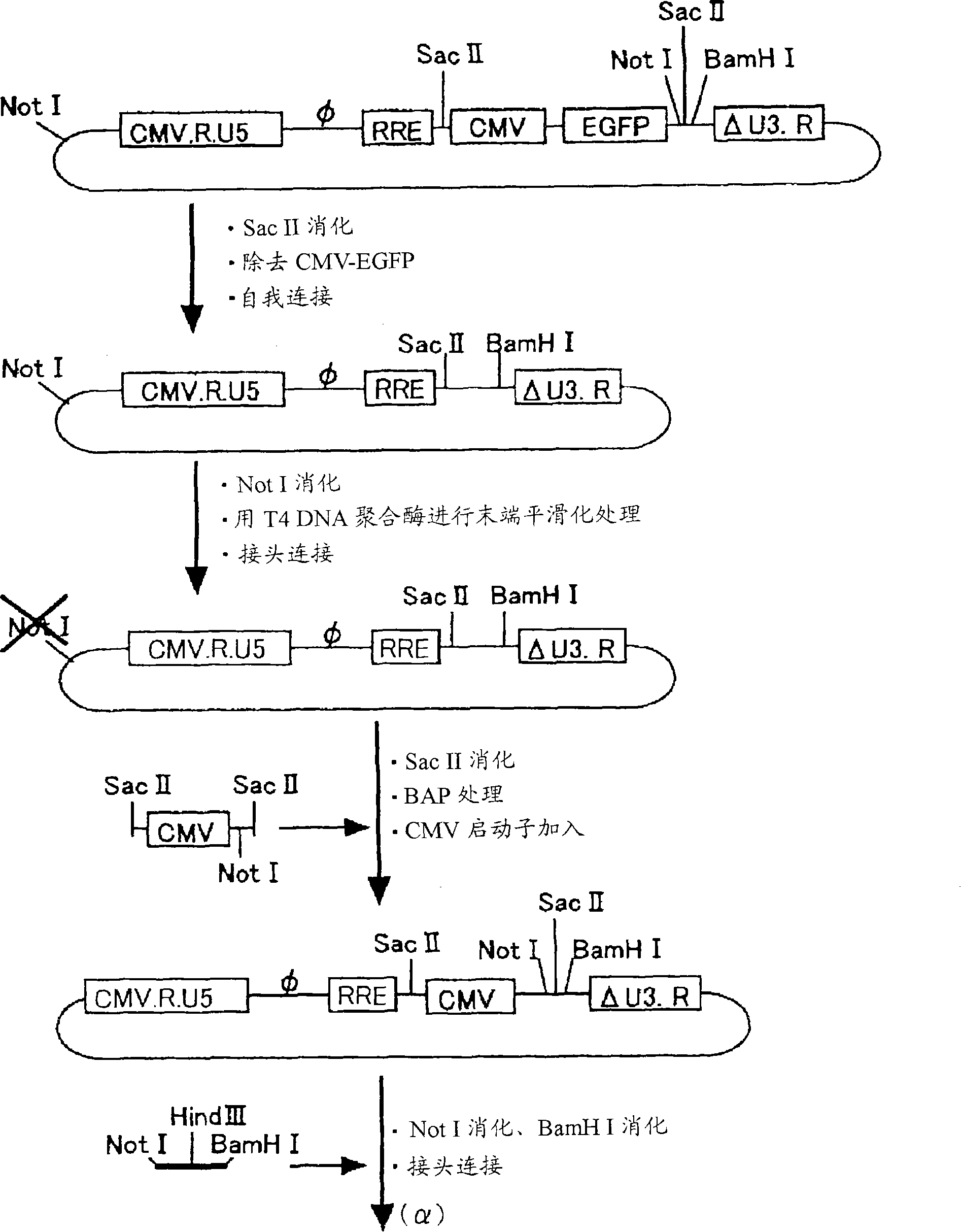
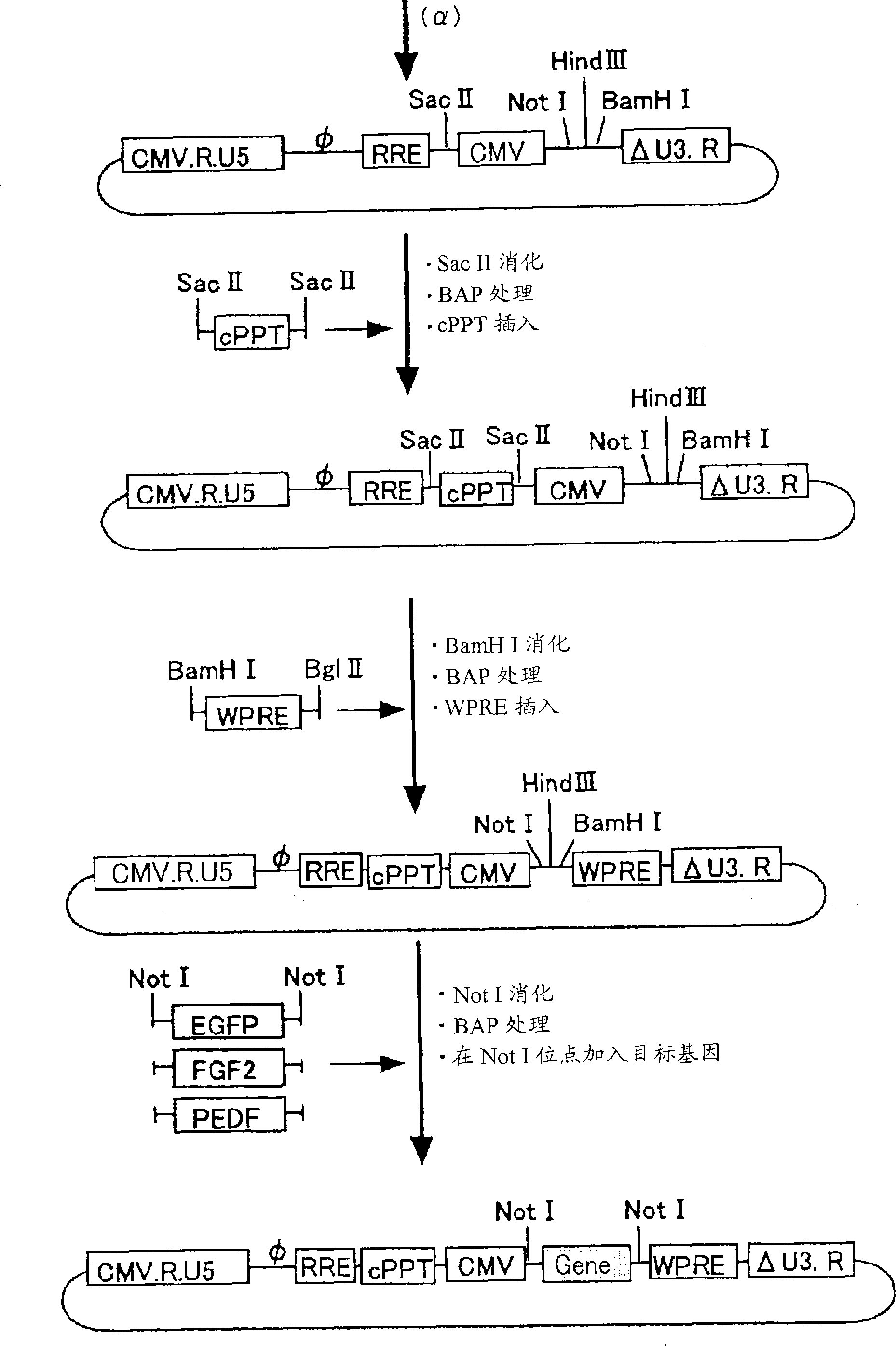
[0099] The vector was constructed using such as figure 1 The 4 plasmids shown in (gene transfer vector, packaging vector, rev expression vector, VSV-G expression vector). Among them, the gene transfer vector, the packaging vector, and the rev expression vector were prepared by modifying the original vector plasmid (PCT / JP00 / 03955). For the VSV-G expression vector, the original vector without modification was used.
[0100] For plasmid preparation, various commercially available kits were used. Restriction endonucleases were produced by New England Biolabs, and plasmid DNA was extracted, purified, and recovered using QIAGEN kits (QIAquick PCR purification kit, QIAquick Nucleotide Removal kit, QIAquick Gel extraction kit, Plasmid Maxi kit). For PCR, TaKaRa's EX Taq enzyme was used, and the primers used were synthesized by an external contractor (SIGMA GENOSYS JAPAN). Dephosphorylation of DNA ends used Takara's a...
Embodiment 2
[0118] Example 2 Functional evaluation of the SIV vector carrying cPPT, WPRE
[0119] In order to investigate the introduction effect of cPPT and WPRE, in addition to carrying cPPT and WPRE at the same time, a vector carrying cPPT alone and WPRE alone was also produced, and compared with the original control. All gene transfer vectors used carried EGFP. The original type (sequence number: 33) was used as the packaging carrier.
[0120] 2-1. Preparation of SIV vector
[0121] The cell line 293T cells from human fetal kidney cells were divided into about 1×10 per 15cm plastic culture dish 7 Inoculate (70-80% density on the next day) and culture in 20 ml of D-MEM medium (Gibco BRL) containing 10% fetal bovine serum for 24 hours. After 24 hours of culture, the medium was replaced with 10 ml of OPTI-MEM medium (Gibco BRL) and used as transfected cells for later use.
[0122] Dissolve 6 μg of gene transfer vector, 3 μg of packaging vector, and 1 μg of VSV-G expression vector in ...
Embodiment 3
[0134] Example 3 Large-scale preparation and concentration of SIV vectors carrying therapeutic genes
[0135] like figure 1 As shown, the SIV vector was prepared as follows on the basis of four kinds of plasmids: a modified gene transfer vector, a packaging vector, a rev expression vector, and a VSV-G expression vector. The vectors carrying the therapeutic genes of PEDF and FGF2 are produced in units of 20 15cm dishes.
[0136] According to each 15cm plastic Petri dish about 1×10 7 The 293T cells were inoculated (at a density of 70-80% on the next day), and cultured in 20 ml of D-MEM medium containing 10% fetal bovine serum for 24 hours. After 24 hours of cultivation, the medium was replaced with 10 ml of OPTI-MEM medium for transfection. Dissolve 10 μg of gene transfer vector, 5 μg of packaging vector, 2 μg of rev expression vector, and 2 μg of VSV-G expression vector in 1.5 ml of OPTI-MEM medium for each culture dish, then add 40 μl of PLUS Reagent reagent (Invitrogen) fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com