Optical activity alpha-amino acid and preparation of derivative thereof
A technology of alkyl and benzyl, which is applied in the field of preparing optically active α-amino acid and α-amino acid derivatives, and can solve the problem of not being able to obtain optically active amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1R2
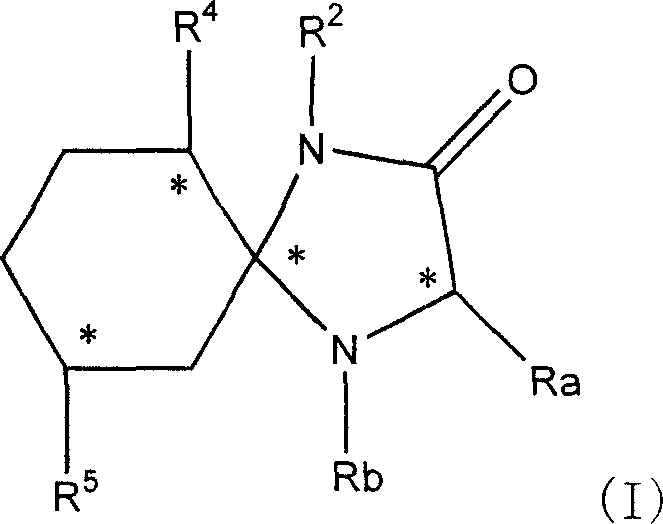
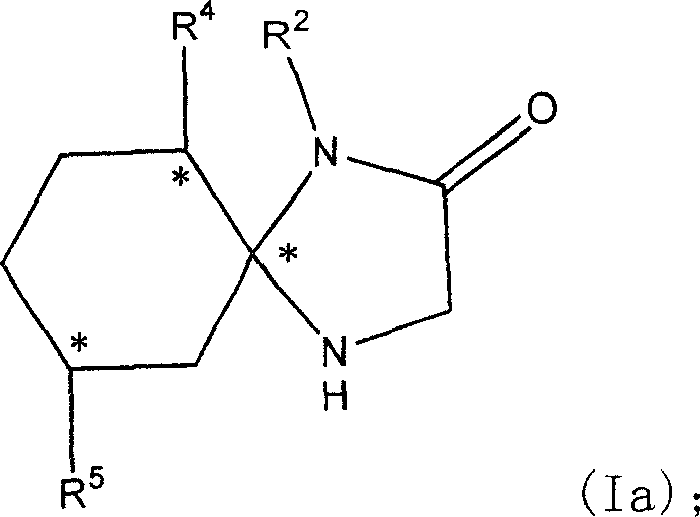
[0162] Example 1R2, R4, R5 is the preparation of H imidazolinone 2, that is, the synthesis of 1,4-diazaspiro[4,5]decan-2-one
[0163] 40 millimoles of cyclohexanone and equimolar glycamide hydrochloride were refluxed in a mixture of methanol and triethylamine for 40 hours. After evaporating the solvent to dryness, the resulting residue was dissolved in ethyl acetate and water, and the aqueous layer was separated and extracted twice more with ethyl acetate. The organic layers were combined, washed with water, saturated brine, and dried over anhydrous sodium sulfate. After removing the desiccant, evaporate to dryness under reduced pressure to obtain white solid product #1 (1,4-diazaspiro[4,5]decan-2-one). (See Table 1)
[0164] Example 2R2 is H, R4 is isopropyl, and R5 is the synthesis of methyl imidazolinone, that is, the synthesis of (5S, 6S, 9R)-6-isopropyl-9-methyl-1, 4-diazaspiro [4,5 ]decan-2-one)
[0165] 10 millimoles of L-menthone and equimolar glycamide hydrochlori...
example 4
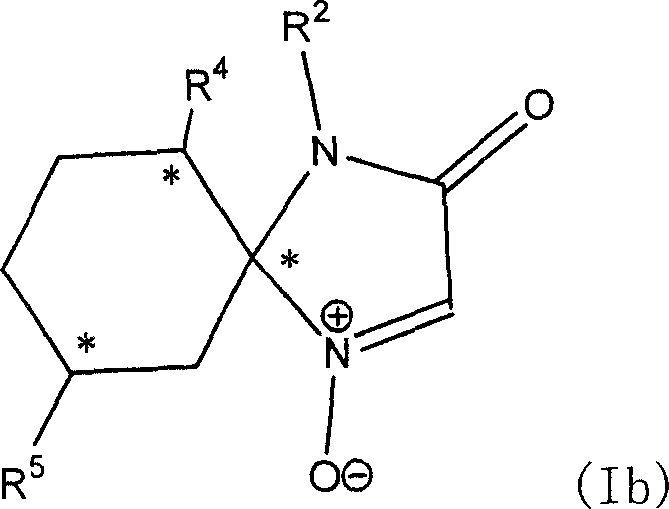
[0171] Example 4 prepares nitrone compound (R2, R4, R5 is H),
[0172] That is, the synthesis of 1,4-diazaspiro[4,5]decan-3-one-1-oxide
[0173]20 millimoles of compound #1 obtained in Example 1 were dissolved in 300 milliliters of dichloromethane, and 50 millimoles of 70% m-chloroperoxybenzoic acid were added several times under the cooling reaction condition in an ice-water bath. Stirring was continued at this temperature for 3 hours after the addition was complete. Excess m-chloroperoxybenzoic acid was eliminated with aqueous sodium sulfite and washed with saturated sodium bicarbonate solution until no gas evolved. The aqueous layer was extracted twice with dichloromethane, and all the collected organic layers were washed once with saturated brine and dried over anhydrous sodium sulfate. After filtration to remove sodium sulfate, evaporation to dryness gave the product #3 as a white solid. (See Table 2)
example 5
[0174] Example 5 prepares nitrone compounds (R2 is H, R4 is isopropyl, R5 is methyl), that is, synthesis of (5R, 6S, 9R)-6-isopropyl-9-methyl-1, 4-diazaspiro [4, 5] decan-3-one-1-oxide
[0175] The #2 compound (prepared by L-menthone) obtained in the example 2 of 10 millimoles is dissolved in 200 milliliters of methylene chloride, under the cooling reaction condition of ice-water bath, divide and add 30 millimoles of 70 % m-chloroperoxybenzoic acid. Stirring was continued at this temperature for 3 hours after the addition was complete. Excess m-chloroperoxybenzoic acid was eliminated with aqueous sodium sulfite and washed with saturated sodium bicarbonate solution until no gas evolved. The aqueous layer was extracted twice with dichloromethane, and all the collected organic layers were washed once with saturated brine and dried over anhydrous sodium sulfate. After filtering off the sodium sulfate, it was evaporated to dryness to give the product #4 as a white solid. (See T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com