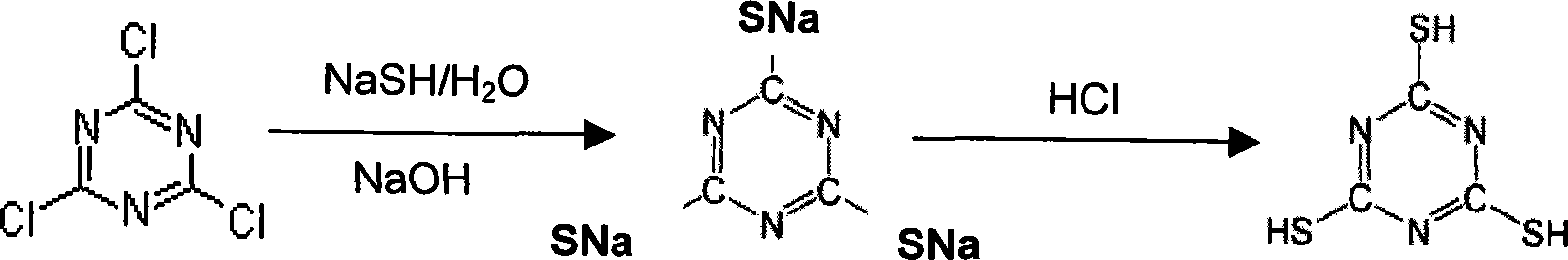
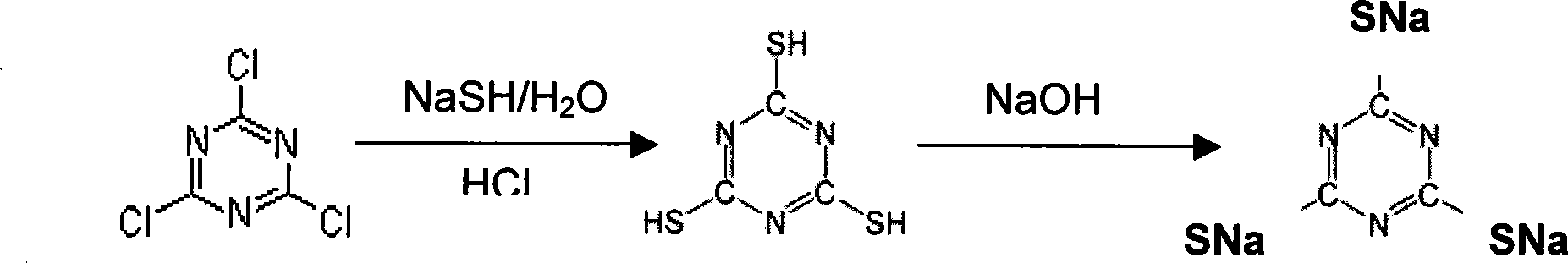
2,4,6- trithione-s-triazine alkali metal salt preparation method
A technology of alkali metal salt and trimercapto, applied in 2 fields, can solve problems such as consumption and low yield, and achieve the effects of low sodium chloride content, high product yield and short preparation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 200 g (1.427 mol) of 40 wt% NaSH aqueous solution was added to 130 ml of water, and the temperature of the solution was adjusted to 50°C. Then, an aqueous solution of 87 g (0.473 mol) of cyanuric chloride and 70 g (0.87 mol) of 50 wt% NaOH was added. At this time, the pH value was maintained at 9.5 and the temperature at 50°C. After all the cyanuric chloride and NaOH had been added, the mixture was stirred at 50°C for 30 minutes.
[0068] After cooling the reaction solution to room temperature, concentrated HCl was added to acidify to pH 6. At this time Na3T-H 3 Quantitative precipitation will occur. While filtering the precipitate according to centrifugation, use a small amount of water to remove a small amount of NaCl doped in the precipitate.
[0069] The weight of the solid obtained by centrifugation was 136.9 g, containing 60 wt% Na3T-H 3 (Being the initial material, being equivalent to 98 mol % of the total amount of cyanuric chloride added), the remainder is ...
Embodiment 2
[0074] In a container containing 150ml of water, add 19.04g of Na 2 After S (0.244 mol) and 60.8 g of NaSH (1.084 mol) were dissolved, 80 g of cyanuric chloride (0.434 mol) was added. At this time the temperature was maintained at 45°C.
[0075] The initial pH value was 9. During the addition of cyanuric chloride, 50 wt% NaOH was added, and the pH value was maintained while stirring for 1.5 hours.
[0076] After cooling the reactant to room temperature, concentrated HCl was added to acidify to pH 6. At this time Na3T-H 3 Quantitative precipitation will occur. While filtering the precipitate according to centrifugation, use a small amount of water to remove a small amount of NaCl doped in the precipitate.
[0077] The weight of the solid obtained by centrifugation is 130g, containing 58wt% Na3T-H 3 (Being the initial material, being equivalent to 98 mol % of the total amount of cyanuric chloride added), the remainder is water.
[0078] 130 g of the solid obtained by centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com