Method for improving secernment efficiency of recombined protein
A recombinant protein and efficiency technology, applied in the biological field to achieve the effect of improving the efficiency of recombinant protein secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Construction of Cell Secreted Expression Vector
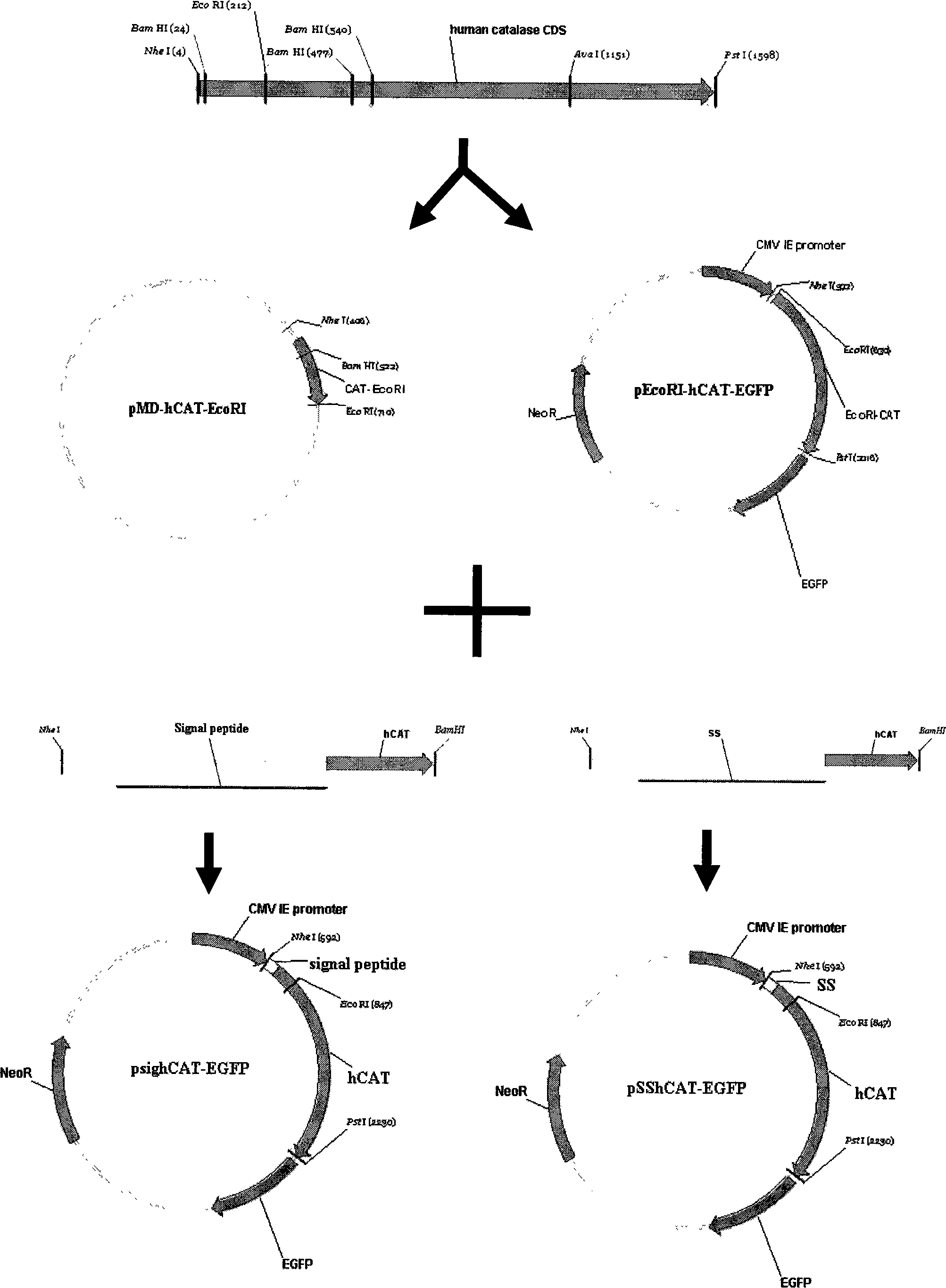
[0028] 1.1 Construction of classic secretion expression vector of human catalase
[0029] The whole construction process of the classic secretion vector is shown in Figure 1, and the details are as follows:
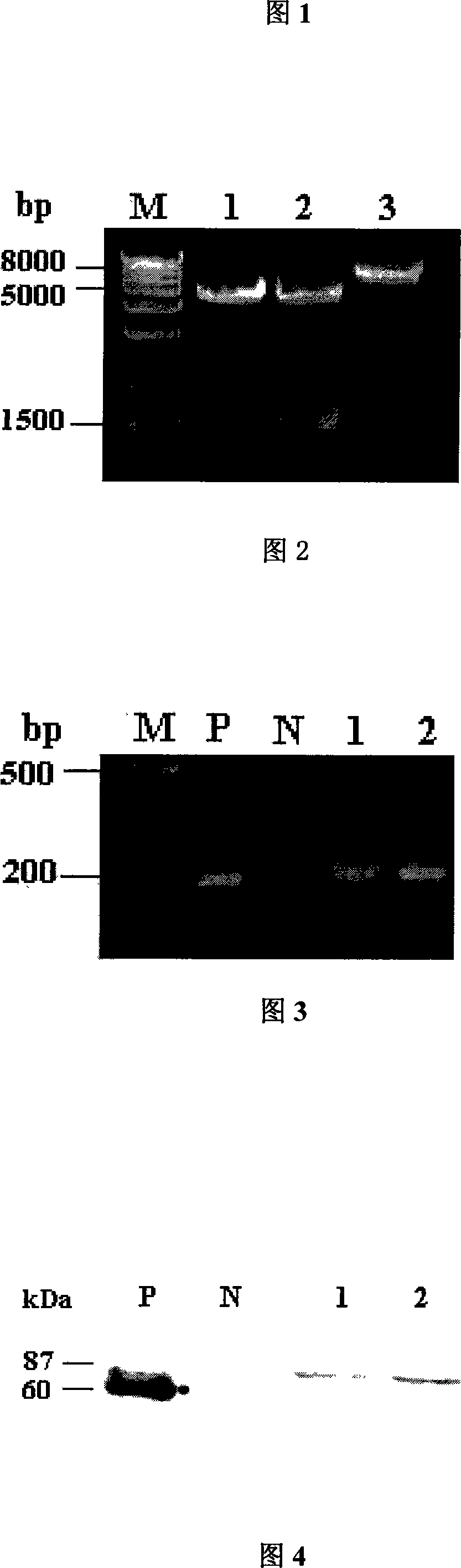
[0030] Using the EST cDNA clone of Invitrogen Company IMAGE number: 4515735 as a template, and based on the full-length cDNA sequence of the human catalase gene (accession number NM 001752) included in NCBI, primers were designed to amplify the sequence of the gene coding region. NheI and PstI restriction sites were introduced into the two ends respectively, and the stop codon of the gene was deleted. The upstream primer is NheI F: 5'-TTGCTAGCAGATGAAGGTCCTCATCC-3', and the downstream primer is PstI R: 5'-TTCTGCAGCAGATTTGCCTTCTCCCCT-3'. The PCR product was recovered and connected to the pMD19-T vector for sequencing. After the sequence was confirmed to be correct, EcoRI and PstI were used for double diges...
Embodiment 2
[0044] Example 2 CHO cell transfection and expression detection
[0045] 2.1 Cell culture and transient transfection
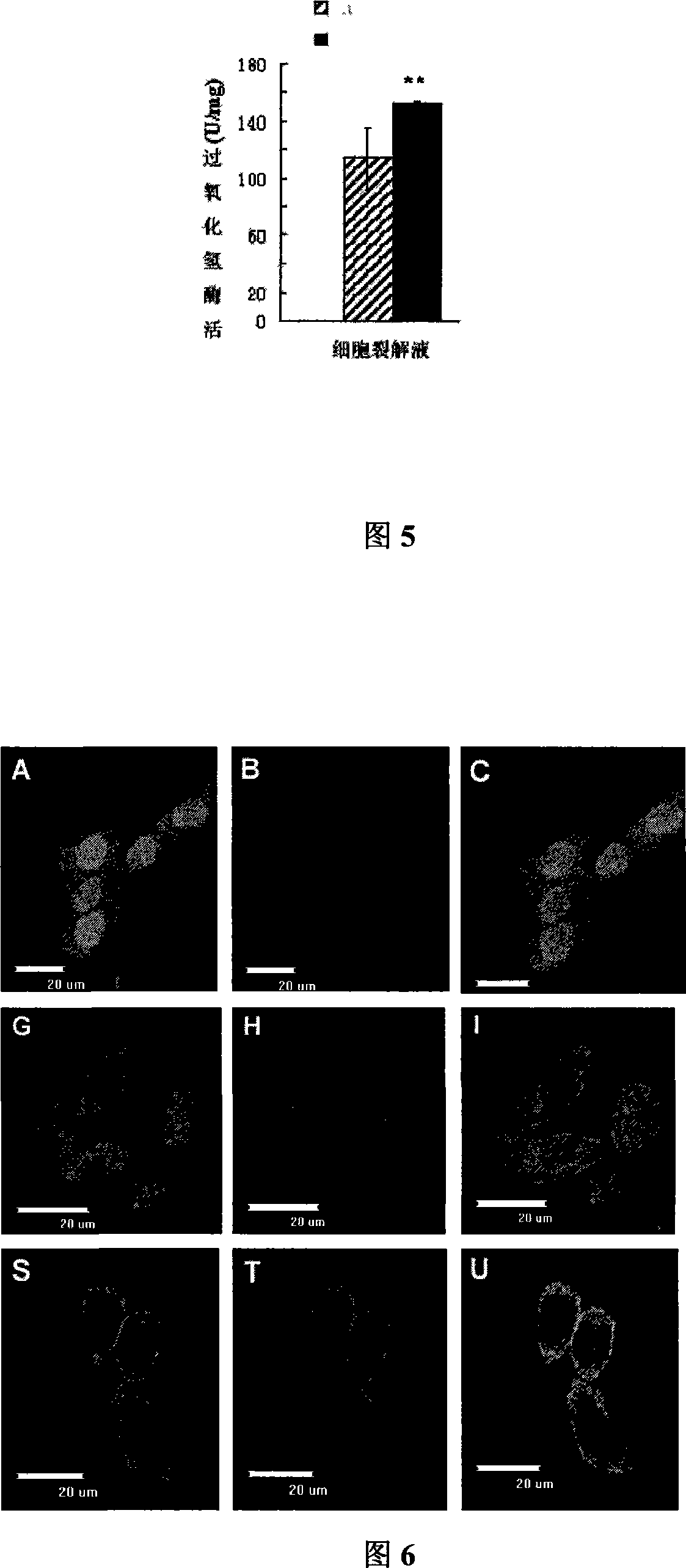
[0046] CHO cells will be planted in 6-well cell culture plates, and when they are cultured in DMEM medium containing 10% fetal bovine serum to 70-80% confluence, according to the instructions, use Lipofectamine 2000 liposomes to secrete the expression vector psighCAT- EGFP and pSShCAT-EGFP and control empty vector pEGFP-N1 were transferred into the cells.
[0047] 2.2 RT-PCR and Western blot detection
[0048] The transfected CHO cells were cultured in a 37°C incubator for 24 hours, the total RNA was extracted with TRIzol, and the residual DNA was removed with DNase, as a template, after reverse transcription with Oligo dT primers to synthesize cDNA strands, the following pair of primers were used to amplify A 184bp fragment in the human catalase gene was added. Upstream primer: hCAT F 5'-TGCTGAATGAGGAACAGAGG-3'; downstream primer: hCAT R5'-GTGTGAATCGCATTCT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com