Cortex moutan valid target pharmaceutical combination, preparation method and application thereof
A technology of effective parts and compositions, applied in the pharmaceutical composition of effective parts of paeonol, its preparation and application, to achieve the effects of fixed main component content and ratio, low cost, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
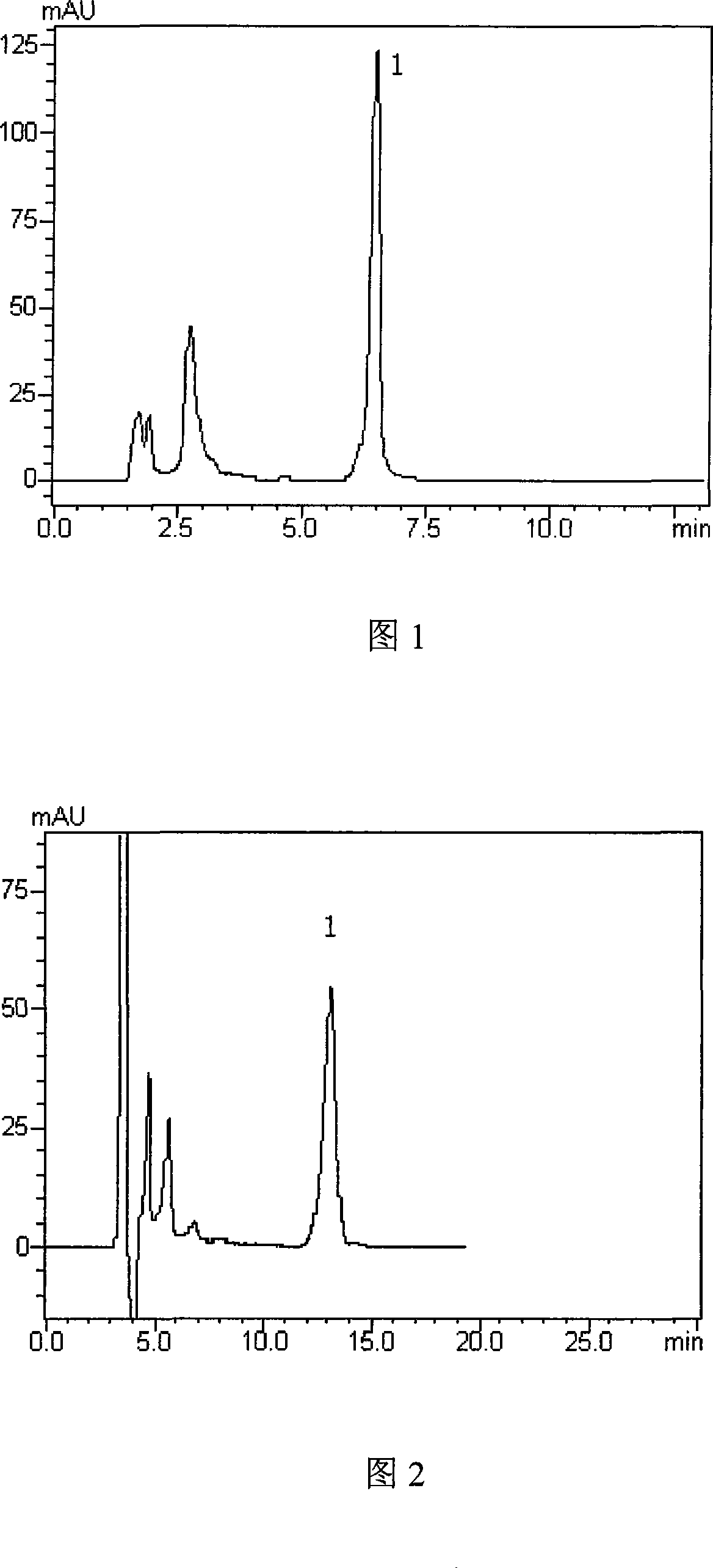
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 paeonol effective fraction composition
[0047] Take paeonol medicinal material 5kg, cut into decoction pieces, put it in a still, add 20L of water to distill, collect 8L of distillate, refrigerate (0-10°C), overnight, filter to obtain 29g of paeonol crystals. Filter the paeonol water infusion in the distiller, and place the filtrate for later use. Take the above filter residue, add 50L water to extract 3 times, each time for 1 hour; combine the extracts, combine the above filtrates, apply macroporous adsorption resin column chromatography, first elute with water, then elute with 90% ethanol, collect 90% ethanol The eluate was concentrated under reduced pressure and dried to obtain 221 g of total paeonol glycosides. Paeonol was mixed with total paeonol glycosides to prepare 250 g of paeonol effective fraction composition, with a yield of 5.0%. It is determined that the total paeonol and total paeonol glycosides contained in the paeonol ...
Embodiment 2
[0049] The preparation of embodiment 2 paeonol effective fraction composition
[0050] Take paeonol medicinal material 5kg, cut into decoction pieces, put into a distiller, add 40L of water to distill, collect 25L of distillate, refrigerate (0-10°C), overnight, filter to obtain 67g of paeonol crystals. Filter the paeonol water infusion in the distiller, and place the filtrate for later use. Take the above filter residue, add 100L 50% ethanol to extract once, and extract for 3 hours; combine the extracts, recover ethanol under reduced pressure, combine with the above filtrate, and perform chromatography on a macroporous adsorption resin column, first elute with water, and then use 80% ethanol For elution, the 80% ethanol eluate was collected, concentrated under reduced pressure, and dried to obtain 260.5 g of total paeonol glycosides. Paeonol was mixed with total paeonol glycosides to obtain 327.5 g of paeonol effective part composition, with a yield of 6.55%. It is determine...
Embodiment 3
[0052] The preparation of embodiment 3 paeonol effective fraction compositions
[0053] Take 5 kg of paeonol medicinal material, crush it into coarse powder, put it in a distiller, add 100 L of water to distill, collect 75 L of distillate, refrigerate (0-10° C.), overnight, and filter to obtain 88 g of paeonol crystals. Filter the paeonol water infusion in the distiller, and place the filtrate for later use. Take the above filter residue, add 40L of water, extract twice, each time for 1 hour; combine the extracts, and combine with the above filtrate, apply macroporous adsorption resin column chromatography, first elute with water, then elute with 70% ethanol, collect The 70% ethanol eluate was concentrated under reduced pressure and dried to obtain 288 g of total paeonol glycosides. Paeonol was mixed with total paeonol glycosides to prepare 376 g of paeonol effective part composition, with a yield of 7.54%. It is determined that the total paeonol and total paeonol glycosides...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com