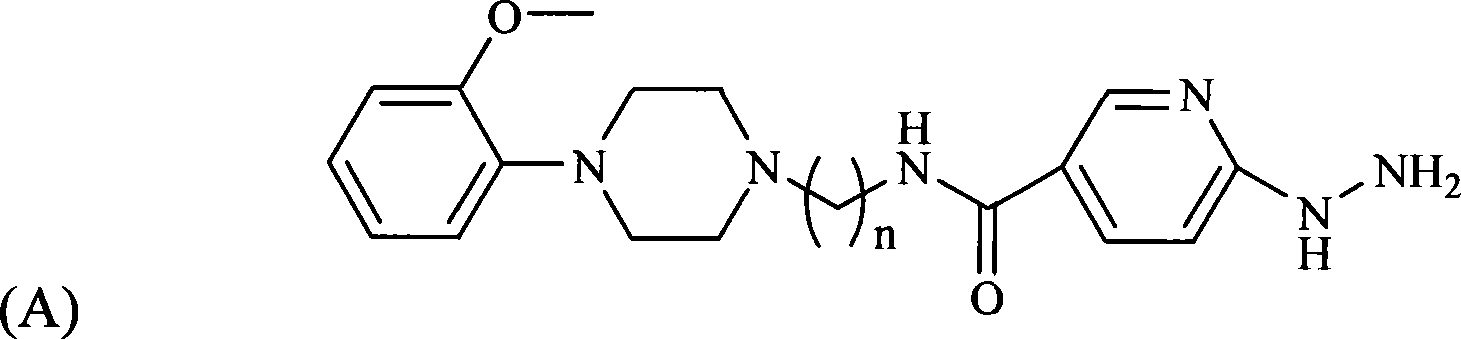99mtc marked 2-methoxyphenyl piperazine derivative complexes as well as preparation method and uses thereof
A technology of methoxyphenyl and 99mtc, which is applied in the field of radioactive complexes, can solve the problems of low brain uptake, strong fat-solubility, and poor specificity of complexes, and achieve simple preparation methods, excellent biological properties, and low cost of preparation and use low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
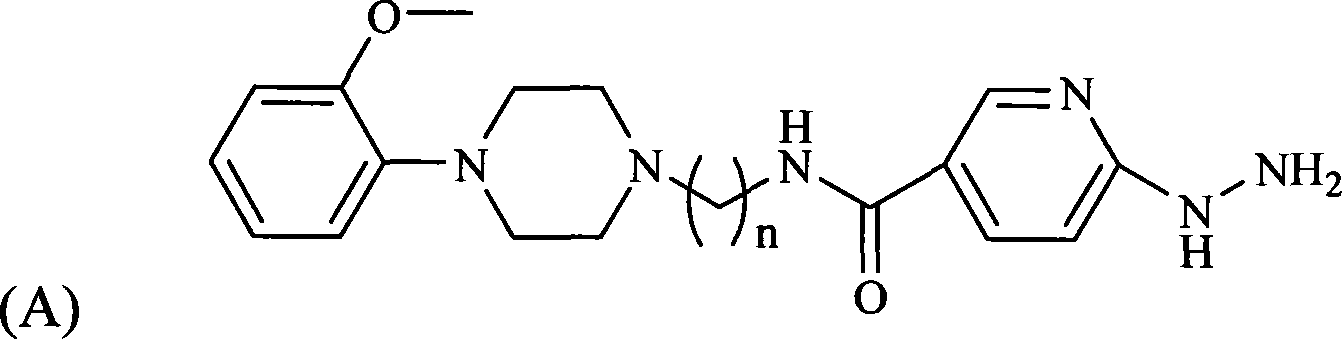
[0058] One of the ligands prepared according to the following steps is the compound HYNIC-MPP3 with n taking 3 shown in formula (A), and the other ligands are HEDTA 99m Tc-labeled 2-methoxyphenylpiperazine complexes:
[0059] 1) Synthesis of HYNIC-MPP3 ligand
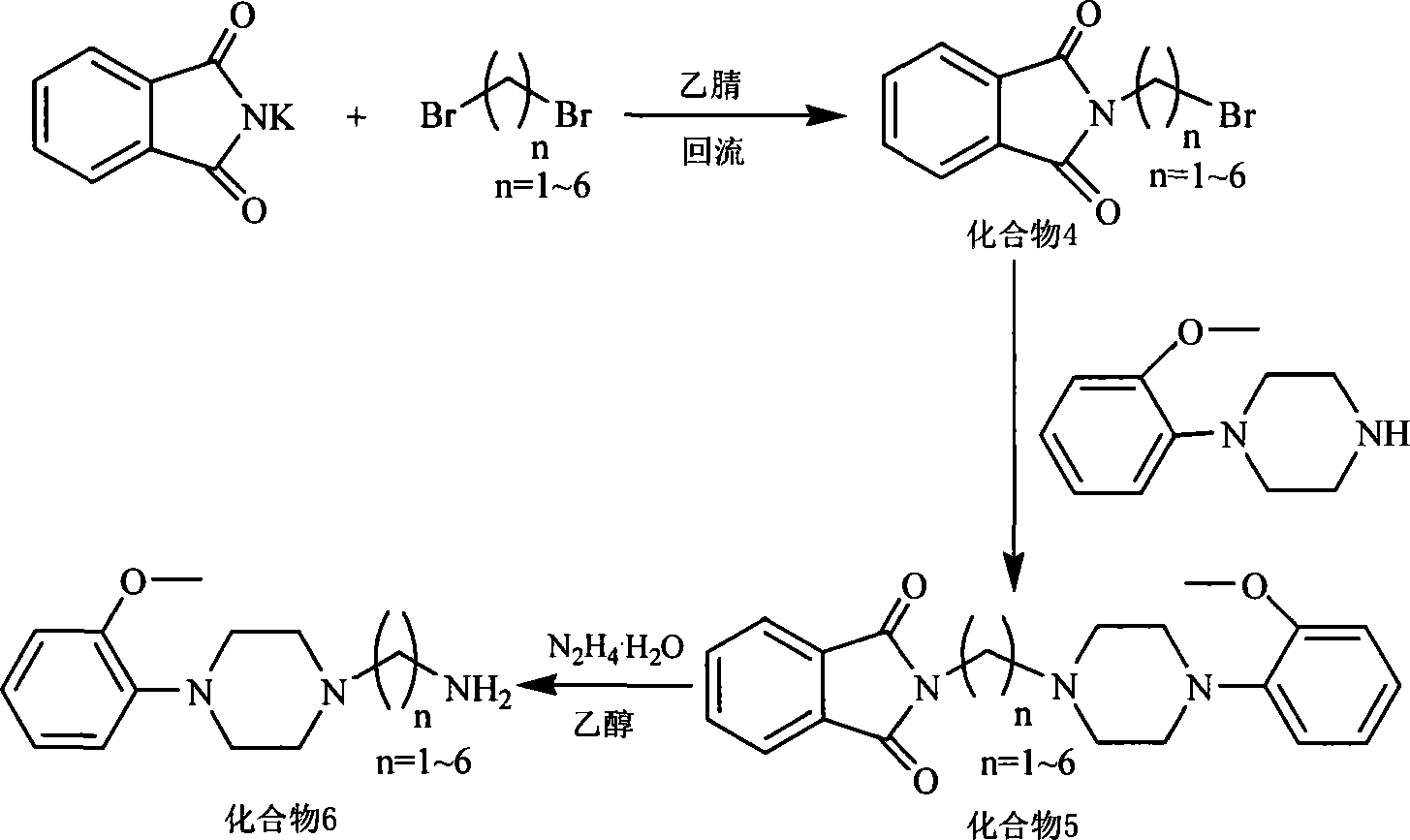
[0060] 1.1) Potassium phthalimide (4.003g, 0.022mol) and 1,3-dibromopropane (11.151g, 0.055mol) were added to a 50mL three-necked flask, heated to reflux in 30mL of acetonitrile for 5 hours , The solution was a white cloudy liquid during the reaction. After the reaction, filter and concentrate the filtrate to obtain a light yellow-green liquid, add 8 mL of methanol, and precipitate about 2.010 g of crystals at -18° C., with a yield of 24%.
[0061] 1.2) Take the crystallization 1.021g (4mmol) obtained in step 1.1), add 0.912g (4mmol) of 2-methoxyl-phenylpiperazine hydrochloride and K in a 50mL three-necked flask 2 CO 3 1.108 g (4 mmol). After refluxing in acetonitrile for a while, the solution turned milky yellow. ...
Embodiment 2
[0076] One of the ligands prepared according to the following steps is the compound HYNIC-MPP3 with n taking 3 shown in formula (A), and the other ligands are tricine 99m Tc-labeled 2-methoxyphenylpiperazine complexes:
[0077] 1) Synthesis of HYNIC-MPP3 ligand
[0078] The ligand HYNIC-MPP3 was synthesized according to the method described in step 1) of Example 1.
[0079] 2) Synthesis of complexes
[0080] 2.1) Prepare the solution according to the method of step 2.1) in Example 1, except that HEDTA as other ligands is replaced with tricine;
[0081] 2.2) Use the reactant solution prepared in step 2.1) of this example to synthesize the complex according to the method in step 2.2) in Example 1, except that the reaction condition after adjusting the pH value of the solution is to react at 60°C for 10 minutes, and the obtained complex Material use 99m Expressed by Tc-tricine / HYNIC-MPP3.
Embodiment 3
[0083] One of the ligands prepared according to the following method is the compound HYNIC-MPP4 with n taking 4 shown in formula (A), and the other ligands are HEDTA 99m Tc-labeled 2-methoxyphenylpiperazine complexes:
[0084] 1) Synthesis of HYNIC-MPP4 ligand
[0085] The ligand was synthesized according to the method described in step 1) of Example 1, except that the 1,3-dibromopropane used therein was replaced by the same mole number of 1,3-dibromobutane.
[0086] 2) Synthesis of complexes
[0087] 2.1) Prepare the solution according to the method of step 2.1) in Example 1, except that the ligand HYNIC-MPP3 used is replaced by the HYNIC-MPP4 ligand prepared in step 1) of this example;
[0088] 2.2) Use the various reactant solutions prepared in step 2.1) of this example to synthesize the complex according to the method in step 2.2) in Example 1, except that the reaction condition after adjusting the pH value of the solution is to react at 40°C for 50 minutes to obtain co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com