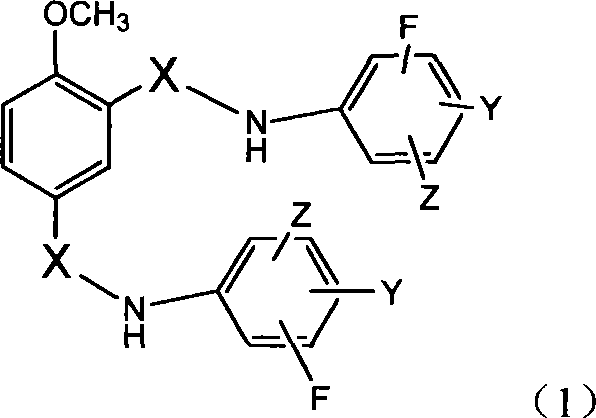
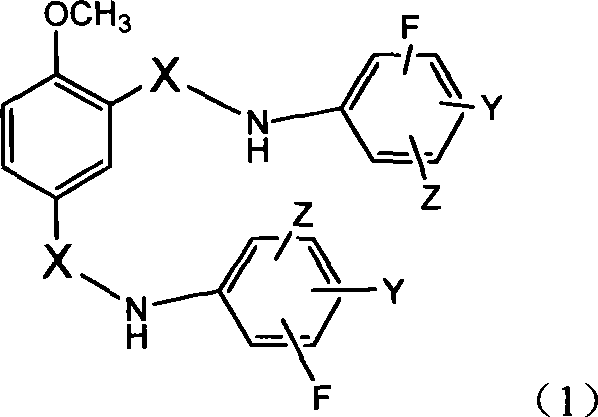
N,N'-difluorophenyl derivative of 4-methoxyl-1,3-phthalamide and use thereof
A technology of phthalamide and difluorophenyl is applied in the preparation of sulfonic acid amide, the preparation of carboxylic acid amide, the preparation of organic compounds, etc., and can solve the problems that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0473] Example 1: 4-methoxy-N, N'-bis-(3,5-difluorophenyl)-1,3-benzenedicarboxamide (C 21 h 14 f 4 N 2 o 3 )Synthesis
[0474] Dissolve 1.3g (10.1mmol) of 3,5-difluoroaniline in 15mL of tetrahydrofuran, add it to a 100mL round bottom flask, and dissolve 1.0g (4.3mmol) of 4-methoxy-1,3-phthaloyl chloride React in 10mL tetrahydrofuran for 6 hours. The solvent was evaporated. The solid was successively recrystallized from ethanol to obtain 1.2 g of white needle-like crystals. Yield: 66.7%, mp: 248-250°C.
[0475] The group number of the compound in the rabbit platelet aggregation reaction experiment is 9i.
[0476] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of 9i: IR(KBr)cm -1 : 3362.5, 3102.9 (υ NH ), 1685.7, 1668.5 (υ C=O ), 1607.9, 1559.7, 1495.9, 1431.1 (υ C=C ), 1311.3(υ C-N ), 1266.6, 1020.2 (υ C-O-C ), 1101.7(υ CF ), 843.6, 828.1, 669.5 (υ CH ); 1 H-NMR (Acetone) δ (ppm): 4.18 (s, 3H, -OCH 3 ...
Embodiment 2
[0477] Example 2: 4-methoxy-N, N'-bis(3,5-difluorophenyl)-1,3-benzenedisulfonamide (C 19 h 14 f 4 N 2 o 5 S 2 )Synthesis
[0478] Dissolve 1.0g (7.8mmol) of 3,5-difluoroaniline in 10mL of tetrahydrofuran, add it to a 100mL round bottom flask, add 1.0g (3.3mmol) of 4-methoxy-1,3-benzenedisulfonyl chloride React in the above-mentioned reaction bottle for 6 hours. The solvent was evaporated. The solid was recrystallized from ethanol to obtain 1.0 g of white crystals. Yield: 61.7%, mp: 212-214°C.
[0479] The group number of the compound in the rabbit platelet aggregation reaction experiment is 10j.
[0480] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of 10j: IR (KBr) cm -1 : 3256.6, 3114.4 (υ NH ), 2953.0 (υ CH3 ), 1626.1, 1488.4, 1438.5, 1410.1 (υ C=C ), 1285.8, 1064.1 (υ C-O-C ), 1350.9, 1185.3, 1163.3 (υ SO2 ), 1309.4(υ C-N ), 993.3(υ CF ), 892.8, 838.8, 708.7 (υ CH ); 1 H-NMR (Acetone) δ (ppm): ...
Embodiment 3
[0481] Example 3: 4-methoxy-N, N'-bis(3-trifluoromethyl-4-fluorophenyl)-1,3-benzenedicarboxamide (C 23 h 14 f 8 N 2 o 3 )Synthesis
[0482] Dissolve 1.0g (5.6mmol) of 3-trifluoromethyl-4-fluoroaniline in 10mL of tetrahydrofuran into a 100mL round-bottomed flask, and add 0.6g (2.6mmol) of 4-methoxy-1,3-benzenedimethoxy The acid chloride was dissolved in 10 mL of tetrahydrofuran and added to the above reaction flask to react for 6 hours. The solvent was evaporated. The solid was recrystallized from ethanol to obtain 1.0 g of taupe solid. Yield: 75.2%, mp: 224-226°C.
[0483] The group number of the compound in the rabbit platelet aggregation reaction experiment is 11k.
[0484] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the 11k structure: IR (KBr) cm -1 : 3357.0(υ NH), 1675.4, 1654.7 (υ C=o ), 1552.0, 1505.3, 1427.2 (υ C=C ), 1329.4(υ CF3 ), 1270.1(υ C-N ), 1250.0(υ C-N ), 1054.2(υ C-O-C ), 1125.0(υ CF ); 1 H-NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com