Method for catalyzing cyclone oxide to synthesizing lactone by using nano magnesium-base catalyst
A technology using magnesium-based catalysts and catalytic oxidation, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of small reaction TON, low repeatability, low catalytic efficiency, etc., to achieve Low cost, high catalytic activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
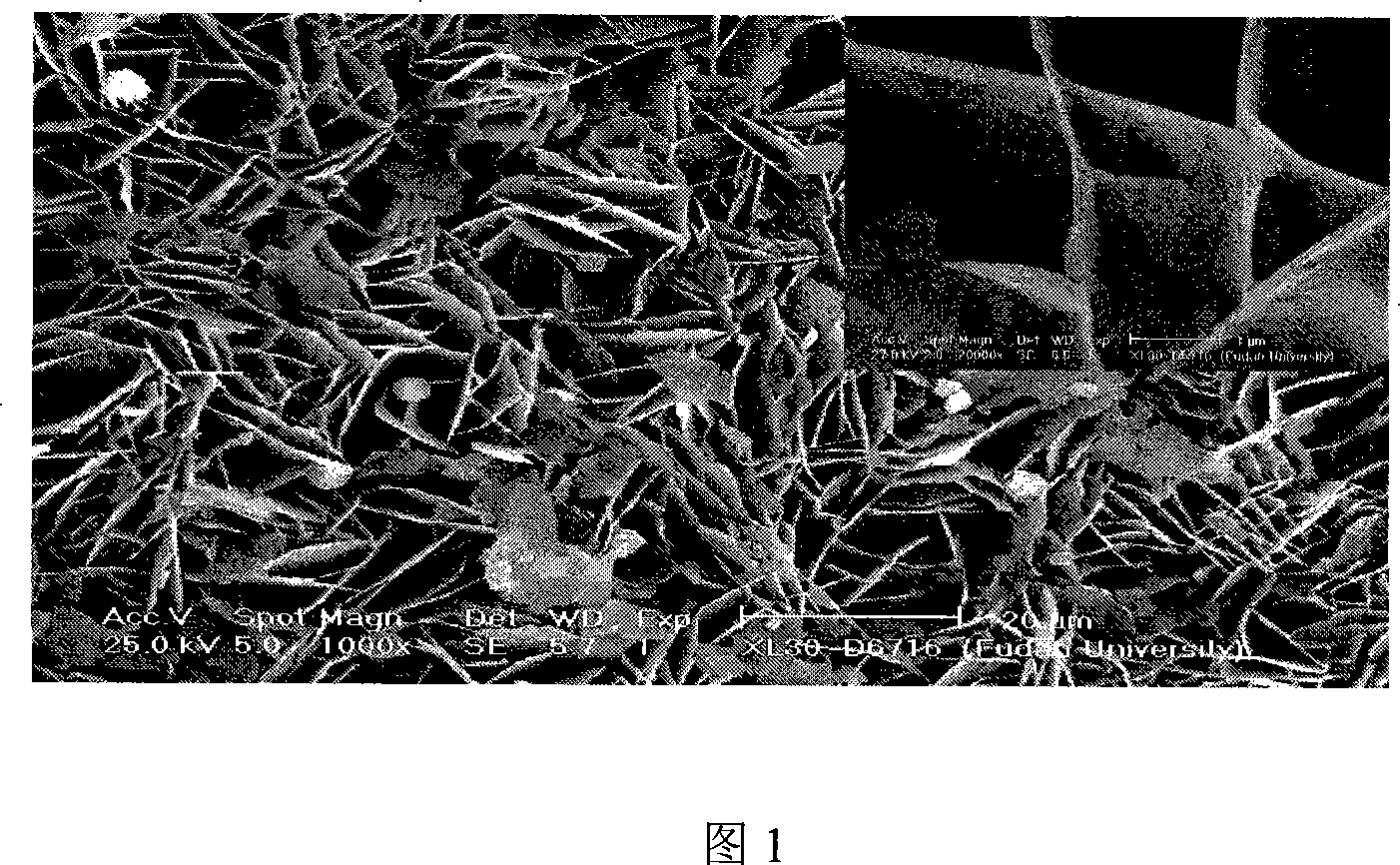
[0016] Embodiment 1: the preparation of sheet-like Mg-based catalyst: 0.02mol MgCl 2 ·6H 2 O and 0.02mol CO(NH 2 ) 2 dissolved in 10mL H 2 O, stirred at 30°C to obtain a clear solution, and added dropwise a volume fraction of 2.5% NH 3 ·H 2 O adjusts pH=8. Stirring was continued for 2 h, and the resulting white suspension was placed in a 200 mL hydrothermal kettle, crystallized at 323 K for 2 h, and suction filtered. The obtained product was washed successively with distilled water and ethanol, dried, and calcined at 373K for 2 hours in an air atmosphere to obtain the catalyst. The reaction conditions adopted are: 0.0029mol cyclohexanone, 2.4mL molar ratio is 1: 0.5 mixed solution of benzonitrile and 1,4-dioxane, 0.9mL of 10% H 2 o 2 , control bath temperature 303K, react for 2 hours, the consumption of catalyst is 0.2 of the mole number of raw material cyclohexanone. Record it as 1#. After the reaction is completed, the analysis results are shown in Table 1.
Embodiment 2
[0017] Embodiment 2: the preparation of flake Mg-based catalyst: 0.04mol MgCl 2 ·6H 2 O and 0.04mol CO(NH 2 ) 2 dissolved in 20mL H 2 O, stirred at 40°C to obtain a clear solution, and added dropwise a volume fraction of 2.5% NH 3 ·H 2 O adjusts pH=9. Stirring was continued for 4 h, and the resulting white suspension was placed in a 200 mL hydrothermal kettle, crystallized at 373 K for 4 h, and suction filtered. The obtained product was washed successively with distilled water and ethanol, dried, and calcined at 473K for 4 hours in an air atmosphere to obtain the catalyst. The reaction conditions adopted are: 0.0029mol cyclohexanone, 2.4mL molar ratio is 1: 1.0 mixed solution of benzonitrile and 1,4-dioxane, 1.2mL of 20%H 2 o 2 , control bath temperature 313K, react for 4 hours, the consumption of catalyst is 0.3 of the mole number of raw material cyclohexanone. Record it as 2#. After the reaction is completed, the analysis results are shown in Table 1.
Embodiment 3
[0018] Embodiment 3: the preparation of sheet-like Mg-based catalyst: 0.08mol MgCl 2 ·6H 2 O and 0.08mol CO(NH 2 ) 2 Dissolve in 30mL H 2 O, stirred at 50°C to obtain a clear solution, and added dropwise a volume fraction of 2.5% NH 3 ·H 2 O adjusts pH=10. Stirring was continued for 6 h, and the resulting white suspension was placed in a 200 mL hydrothermal kettle, crystallized at 423K for 6 h, and suction filtered. The obtained product was washed successively with distilled water and ethanol, dried, and calcined at 573K for 6 hours in an air atmosphere to obtain the catalyst. The reaction conditions adopted are: 0.0029mol cyclohexanone, 2.4mL molar ratio is 1: 1.5 mixed solution of benzonitrile and 1,4-dioxane, 1.5mL of 30%H 2 o 2 , control bath temperature 323K, react for 6 hours, the consumption of catalyst is 0.4 of the mole number of raw material cyclohexanone, denoted as 3#. After the reaction is completed, the analysis results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com