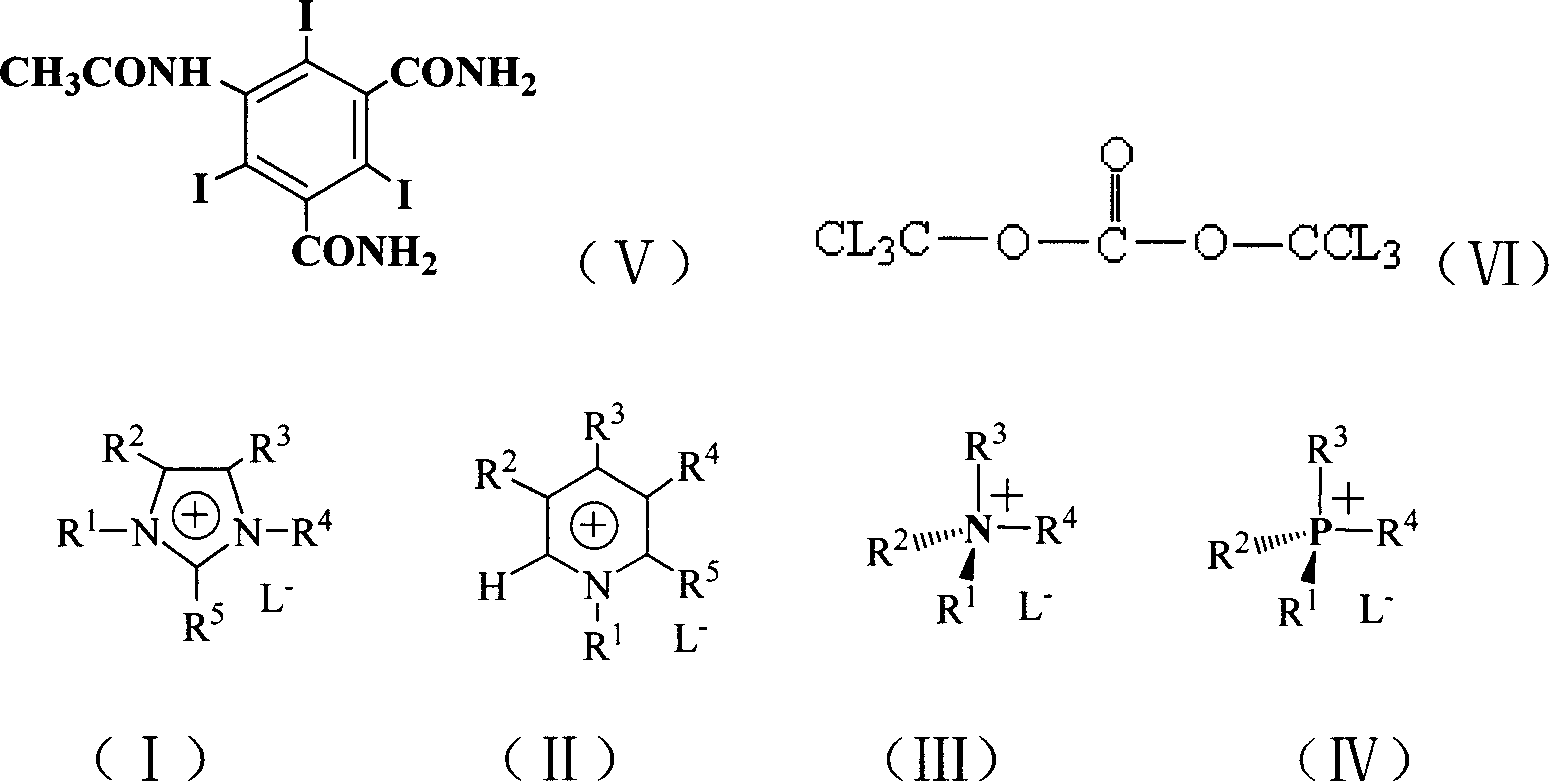Method for preparing 5-acetamido-2,4,6-triiodo-1,3-benzenedicarboxamides
A technology of phthalamide and acetamido, applied in the field of 5-acetamido-2, can solve problems such as environmental pollution, and achieve the effects of less environmental pollution, high yield and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
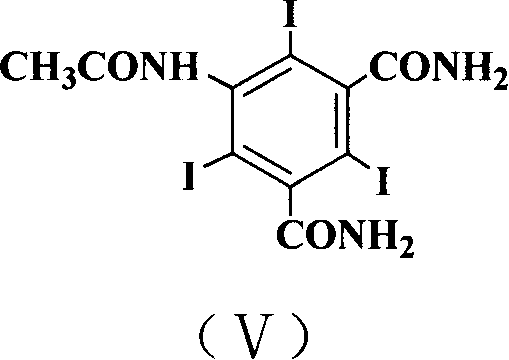
[0023] In the 1000 milliliter reaction bottle, add 5-acetamido-2,4,60 grams (0.1 moles) of triiodoisophthalic acid, 8 grams (0.1 moles) of catalyst pyridine, 12.8 grams (0.04 moles) of solid phosgene, 100 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, heated up to 100°C, stirred, and reacted for 4 hours. After the reaction, cooled to below room temperature, 20 ml of ammonia water (25%) was added dropwise while stirring, Raise the temperature to 60°C, stir for 3 hours, after the reaction is complete, cool to room temperature, filter, and discard the filtrate to obtain 48 grams of the product 5-acetamido-2,4,6-triiodo-1,3-benzenedicarboxamide, melting point > 300 °C, yield 80%. The filtrate obtained by filtration was extracted with dichloromethane, and the dichloromethane layer was taken to evaporate the solvent to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was used for recycling.
Embodiment 2
[0025] In the 1000 milliliter reaction bottle, add 5-acetamido-2,4,60 grams (0.1 moles) of triiodoisophthalic acid, 8 grams (0.1 moles) of catalyst pyridine, 12.8 grams (0.04 moles) of solid phosgene, 100 ml of 1-ethyl-3-methylimidazolium tetrafluoroborate was heated up to 100° C., stirred, and reacted for 4 hours. After the reaction was completed, it was cooled to below room temperature, and 20 ml of ammonia water (25%) was added dropwise while stirring. Raise the temperature to 60°C and stir for 3 hours. After the reaction is complete, cool to room temperature and filter to obtain 48 grams of the product 5-acetylamino-2,4,6-triiodo-1,3-benzenedicarboxamide with a melting point of >300°C. Yield 80%. Extract the filtrate obtained by filtration with dichloromethane, take the dichloromethane layer and evaporate the solvent to obtain 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid, which is used for recycling.
Embodiment 3
[0027] In the 1000 milliliter reaction bottle, add 5-acetamido-2,4,60 grams (0.1 moles) of triiodoisophthalic acid, 16 grams (0.2 moles) of catalyst pyridine, 38.4 grams (0.12 moles) of solid phosgene, 100 ml of 1-butyl-3-methylimidazolium hexafluorophosphate, heated up to 100°C, stirred, and reacted for 4 hours. After the reaction, cooled to below room temperature, 50 ml of ammonia water (25%) was added dropwise with stirring, and heated Stir at 60°C for 3 hours, after the reaction is complete, cool to room temperature, filter, and discard the filtrate to obtain 50 grams of the product 5-acetylamino-2,4,6-triiodo-1,3-phthalamide, melting point > 300°C , yield 83%. Extract the filtrate obtained by filtration with dichloromethane, take the dichloromethane layer and evaporate the solvent to obtain 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid, and the ionic liquid is used for recycling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com