Method for producing amido nickel sulphonic acid
A technology of nickel sulfamate and sulfamic acid, applied in the direction of sulfamic acid, nitrogen and non-metallic compounds, etc., can solve the problems of difficult washing of sodium sulfate, low main grade of products, high cost, etc., and achieve easy washing and reduce Effects of production cost and productivity increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
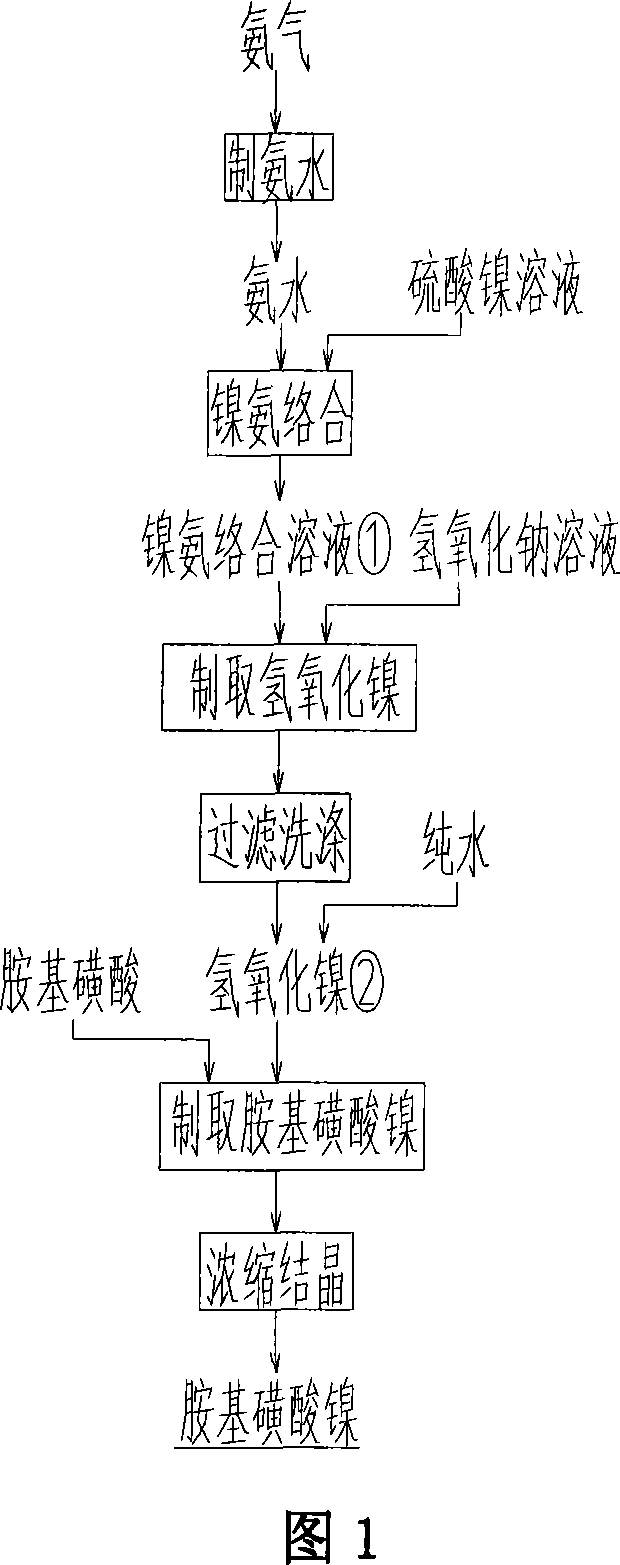
[0032] 1. Use nickel sulfate or nickel chloride solution with a concentration of 80g / L, add saturated ammonia water at a temperature below 50°C, stop ammonia flow when the ammonia content of the complex solution generated in the reaction solution is 25-35g / L gas, to obtain blue nickel-ammonia complex solution ①;
[0033] 2. According to process flow chart 1, get nickel-ammonia complex solution 1. and sodium hydroxide solution, the ratio of the two is nickel: the mol ratio of sodium hydroxide is 1: 2, under the temperature condition below 80 ℃, constantly stir and mix , to obtain nickel hydroxide slurry ①, filter cake ① and filtrate ① are obtained after filtering and washing, and filter cake ① is slurried with pure water to obtain slurry ②; sulfamic acid is prepared into 2mol / The sulfamic acid solution of L; Filtrate 1. returns to reclaim ammonia, reuses;
[0034] 3. Put the slurry obtained in process 2 ② and 2mol / L sulfamic acid solution in a molar ratio of 1:1, and slowly a...
Embodiment 2
[0037] Adopt purified nickel sulfate solution (80g / L), saturated ammonia and industrial sodium hydroxide (120g / L), for subsequent use;
[0038] 1. Get above-mentioned nickel sulfate solution 1L (80g / L), add saturated ammonia water at 50 ℃, when the ammonia content of complexing solution generated in the reaction solution is 25-35g / L, stop flowing ammonia gas, obtain blue color Nickel-ammonia complex solution ①, the time of adding ammonia water is 2 minutes at this time, the volume is increased to 1.5 times, and the nickel ion concentration is 52.6g / L.
[0039] 2. Take 1.5L of nickel-ammonia complex solution, add about 0.9L of industrial sodium hydroxide (120g / L) at an even speed, the solution gradually becomes green suspension, stir constantly, stop adding alkali when the pH reaches 8-8.5, and filter to obtain Nickel hydroxide slurry, the filtrate is treated separately to recover nickel, the filter cake is washed with pure water, and then the obtained filter cake is slurried w...
Embodiment 3
[0043]1. Use the nickel sulfate or nickel chloride solution that concentration is 80g / L, add saturated ammoniacal liquor at the temperature condition of 40 ℃, stop feeding ammonia when the ammonia content of complex solution generated in the reaction solution is 30g / L, obtain Blue nickel ammonium complex solution ①;
[0044] 2. According to process flow chart 1, get nickel-ammonia complex solution 1. and sodium hydroxide solution, the ratio of the two is nickel: the mol ratio of sodium hydroxide is 1: 2, under the temperature condition below 80 ℃, constantly stir and mix , to obtain nickel hydroxide slurry ①, after filtering and washing, filter cake ① and filtrate ① are obtained, and the filter cake ① is slurried with pure water to obtain slurry ②; sulfamic acid is prepared into 2mol / The sulfamic acid solution of L; Filtrate 1. returns to reclaim ammonia, reuses; Its reaction process is:
[0045] 3. Put the slurry obtained in process 2 ② and 2mol / L sulfamic acid solution in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com