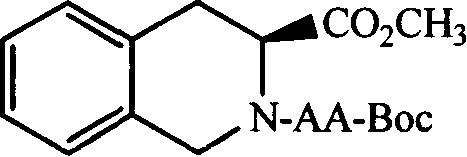
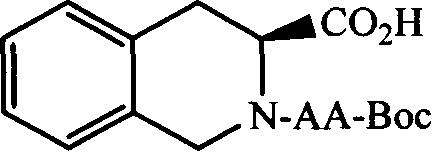
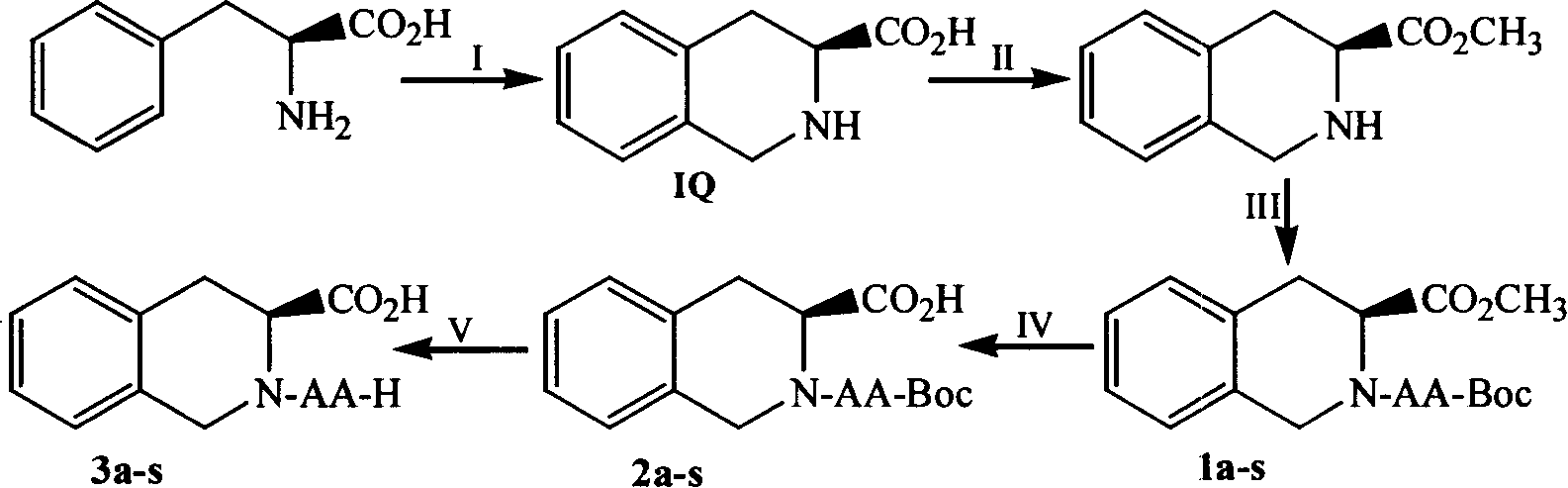
(3S)-N-(L-aminoacyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, preparation method and uses thereof
An alanyl and glycyl-based technology, applied in the field of biomedicine, can solve the problems of low solubility, poor solubility, complex preparation process, etc., and achieve good thrombolytic activity, good antithrombotic activity, and bioavailability high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0044]To 5.0g (0.03mmol) L-phenylalanine, first add 27ml formaldehyde dropwise, and then add 45ml concentrated hydrochloric acid dropwise. The resulting suspension was heated in an oil bath to 80-90°C and stirred for 10 h, TLC (CCl 3 :CH 3 OH=10:1) showed the disappearance of phenylalanine. The reaction mixture was cooled to room temperature and filtered. The filter cake was washed with water (30ml×3) and then with acetone (30ml×3) to obtain 4.5g (83.9%) of the title compound as a colorless solid. Mp.302-303; ESI-MS (m / e) 178 [M+H] + ; 1 HNMR (CDCl 3 -d) δ / ppm=11.0(s, 1H), 7.0(m, 4H), 3.80(m, 3H), 3.03(d.1H), 2.78(d, 1H), 2.0(s, 1H); 13 C-NMR (CDCl 3 -d) δ / ppm=174.9, 136.2, 134.2, 127.2, 126.0, 57.6, 47.4, 29.4.
Embodiment 2
[0045] Example 2 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester
[0046] Add 5.2ml of thionyl chloride dropwise to 20ml of methanol in an ice-salt bath, and stir for 10min after the dropping. Afterwards, 4.27 g (20 mmol) of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid were added. The reaction mixture was stirred at room temperature for 100 h, TLC (CCl 3 :CH 3 OH=5:1) showed the disappearance of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in 20 ml of methanol and concentrated under reduced pressure. This operation was repeated 3 times. The residue was dissolved in 20 ml of ether and concentrated under reduced pressure. This operation was repeated 3 times. The residue was crystallized from methanol / ether to afford 4.2 g (92%) of the title compound as a colorless solid. ESI-MS(m / e)192[M+H] + ; 1 HNMR (CDCl 3 -d) δ / ppm=7.0(m, 4H)...
Embodiment 3
[0047] Example 3 Preparation of (3S)-N-(Boc-L-alanyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester (1a) 0.832g (4.4mmol) Boc-Ala-OH was dissolved in 10 ml tetrahydrofuran (THF). To the resulting solution was added 0.594 g (4.4 mmol) of N-hydroxybenzotriazole (HOBt) under ice cooling. Stir. Mix 0.91g (4.0mmol) (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid methyl ester with 5ml THF first, then adjust the pH to 8-9 with triethylamine, and stir for 30min Then it was mixed with the newly obtained Boc-Ala-OH solution under ice bath, and then 1.071 g (5.2 mmol) of dicyclohexylcarbodiimide (DCC) was added. The reaction mixture was stirred in ice bath for 2 h and at room temperature for 12 h. TLC (ethyl acetate:petroleum ether=1:2) showed that methyl (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylate disappeared. Dicyclohexylurea (DCU) was filtered off, and the filtrate was subconcentrated to dryness. The residue was dissolved with 50ml ethyl acetate, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com