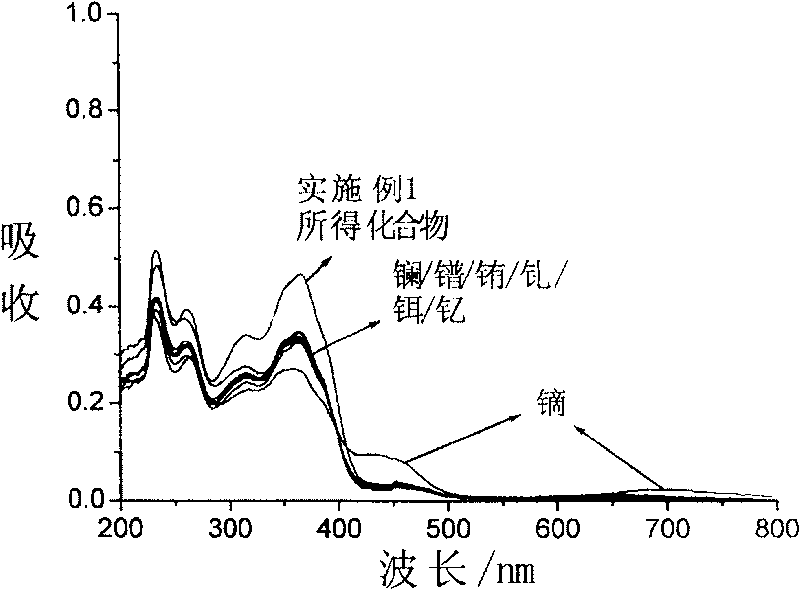
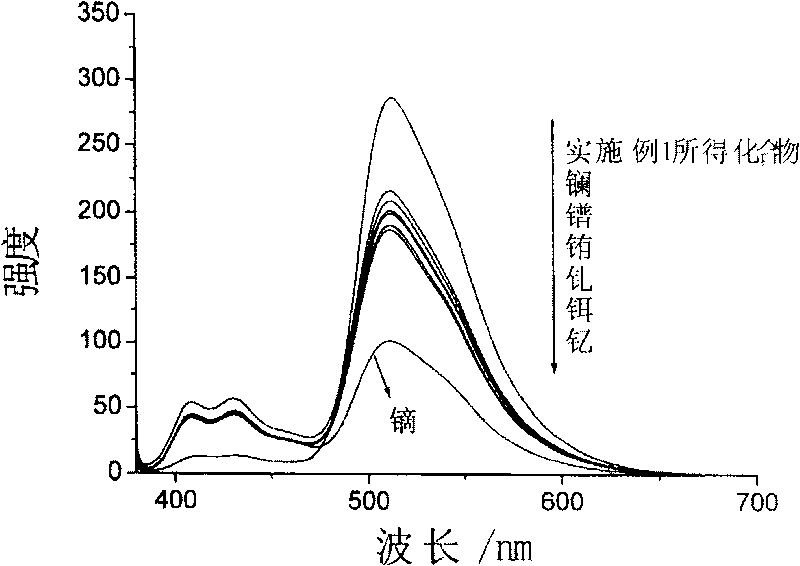
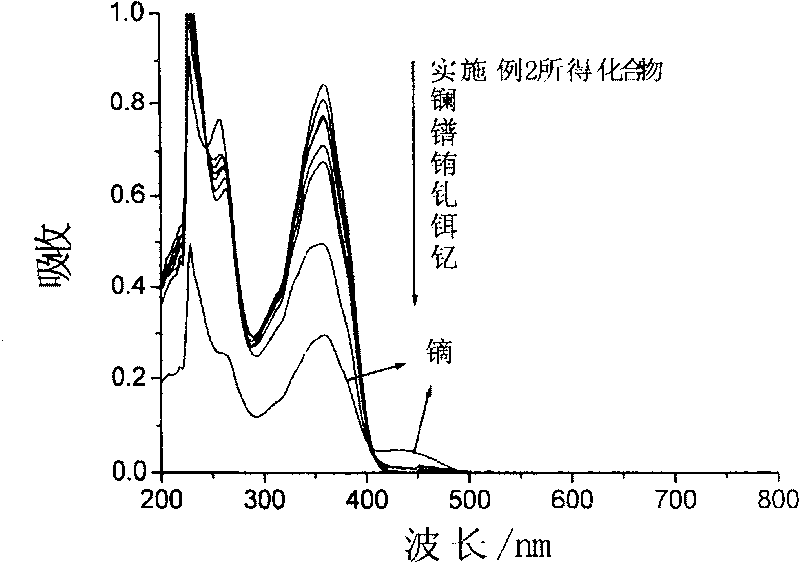
Compound for identifying rare earth metallic ion dysprosium or erbium and its synthesis method and use
A rare earth metal ion and compound technology, applied in chemical method analysis, imino compound preparation, chemiluminescence/bioluminescence, etc., can solve problems such as low efficiency, achieve strong analytical signal, good photochromic properties, and special recognition performance. the effect of one
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 5,17-(5,5'-dinitrosalicylaldehyde acetal)-diimino-25,27-di-n-propoxy-26,28-dihydroxycalix[4]arene
[0026] Diaminocalix[4]arene derivative (0.12g, 0.22mmol), 5-nitrosalicylaldehyde (0.07g, 0.44mmol) and 15ml of absolute ethanol were mixed, and heated to reflux at 70-80°C for 8 hours. The progress of the reaction was monitored by thin-layer chromatography, and the reaction was stopped when there was no starting material. Suction filtration yielded a brown-yellow solid. Recrystallization from dichloromethane / methanol gave pure product (80%). mp>300℃; IR(KBr)v max / cm -1 : 1623 (C=N), 1523, 1337 (NO 2 ); 1 H-NMR (CDCl 3 ),δ H : 14.92 (br, 2H, OH sal), 8.75 (s, 2H, OH), 8.67 (s, 2H, C=N), 8.38-8.34 (d, J=12Hz, 2H, Ar-Hsal), 8.25- 8.21(2d, J=12Hz, 2H, Ar-Hsal), 7.16(s, 2H, Ar-H), 7.09(s, 2H, Ar-Hsal), 7.06-7.01(t, J=15Hz, 4H, Ar -H), 6.88-6.83 (t, J=15Hz, 2H, Ar-H), 4.32-4.27, 3.54-3.49 (2d, J=15Hz, 4H, Ar- CH 2 -Ar), 4.06-4.01(t, J=15Hz, 4H, CH 3 -CH...
Embodiment 2
[0027] Example 2: 5,17-disalicylaldehyde imino-25,27-di-n-propoxy-26,28-dihydroxycalix[4]arene
[0028] Mix diaminocalix[4]arene derivative (0.12g, 0.22mmol), salicylaldehyde (0.055g / 0.05ml, 0.44mmol) and 15ml of absolute ethanol, and heat to reflux at 70-80 degrees Celsius for 8 hours. The progress of the reaction was monitored by TLC, and the reaction was stopped when there was no starting material. Suction filtration yielded a brown-yellow solid. Recrystallization from dichloromethane / methanol gave pure product (80%). mp>300℃; IR(KBr)v max / cm -1 : 1623(C=N); 1 H-NMR (CDCl 3 ),δ H : 13.72 (br, 2H, OH sal), 10.46 (s, 2H, OH), 8.59 (s, 2H, C=N), 7.41-7.32 (m, J=27Hz, 6H, Ar-H sal), 7.10 (s, 4H, Ar-H), 7.05-7.00(t, J=15Hz, 4H, Ar-H), 6.95-6.90(t, J=15Hz, 2H, Ar-H), 6.86-6.81(t, J=15Hz, 2H, Ar-H), 4.38-4.34, 3.47-3.42 (2d, J=15Hz, 4H, Ar- CH 2 -Ar), 4.04-4.00(t, J=12Hz, 4H, CH 3 -CH 2 - CH 2 -O-), 2.12-2.10 (q, J=15Hz, 4H, CH 3 -CH 2 - CH 2 -O-), 1.37-1.32 ...
Embodiment 3
[0029] Example 3: 5,17-(5,5'-dimethoxy salicylaldehyde acetal)-diimino-25,27-di-n-propoxy-26,28-dihydroxycalix[4]arene
[0030] Mix diaminocalix[4]arene derivative (0.12g, 0.22mmol), 5-methoxy salicylaldehyde (0.067g / 0.056ml, 0.44mmol) and 15ml of absolute ethanol, and heat to reflux at 70-80 degrees Celsius 8 hours. The progress of the reaction was monitored by thin-layer chromatography, and the reaction was stopped when there was no starting material. Suction filtration yielded a brown-yellow solid. Recrystallization from dichloromethane / methanol gave pure product (80%). mp>300℃; IR(KBr)v max / cm -1 : 1623 (C=N), 1053, 1239 (CH 3 ); 1 H-NMR (CDCl 3 ),δ H : 13.10 (s, 2H, OH sal), 10.67 (s, 2H, OH), 8.59 (s, 2H, C=N), 7.13-7.11 (d, J=6Hz, 4H, Ar-H), 7.03- 7.00(d, J=9Hz, 4H, Ar-H), 6.98-6.97(d, J=3Hz, 2H, Ar-H sal), 6.94-6.92(d, J=6Hz, 4H, Ar-H sal) , 6.86-6.83 (t, J=9Hz, 2H, Ar-H), 4.38-4.34, 3.47-3.42 (2d, J=15Hz, 4H, Ar- CH 2 -Ar), 4.04-4.00(t, J=12Hz, 4H, CH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com