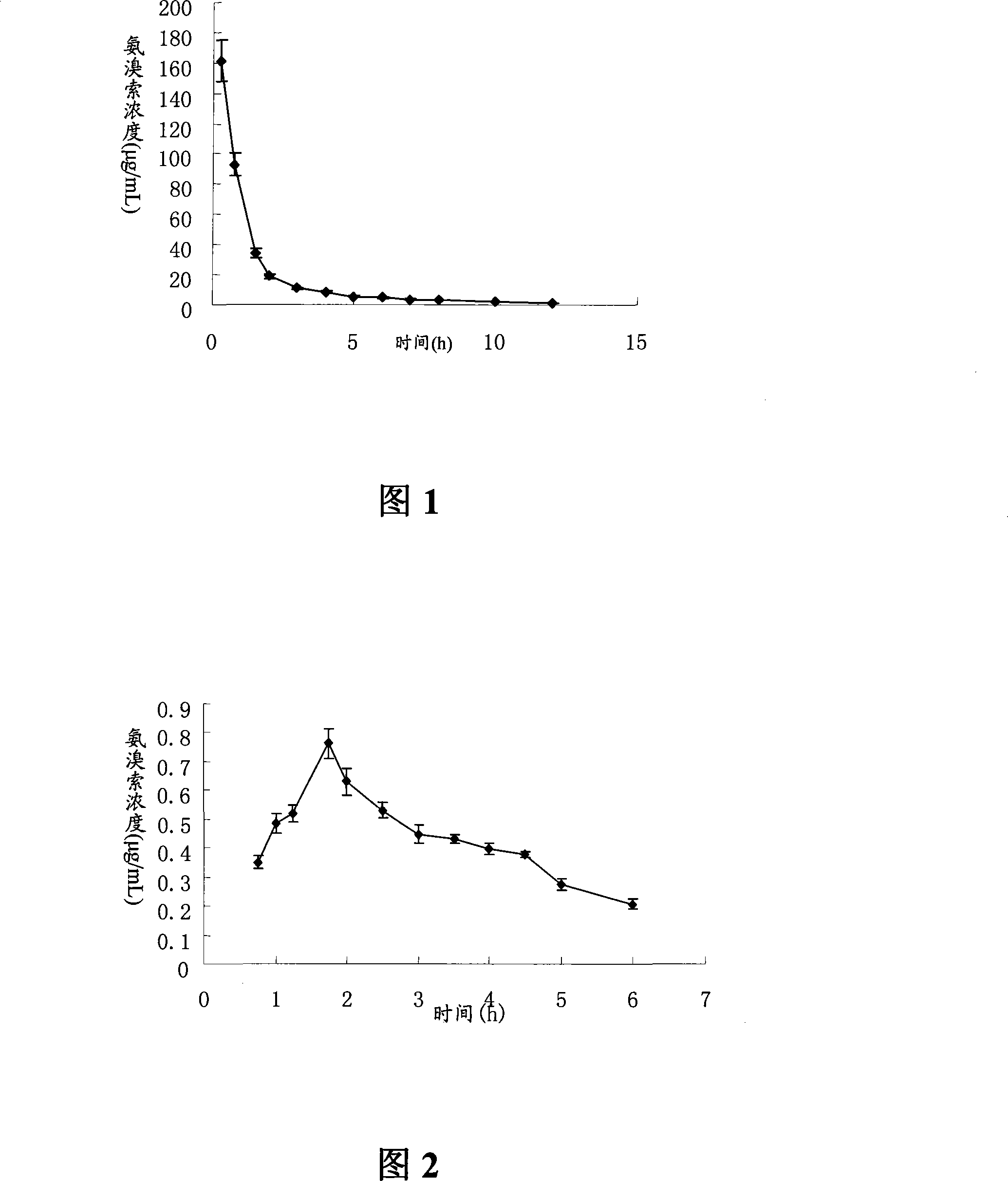
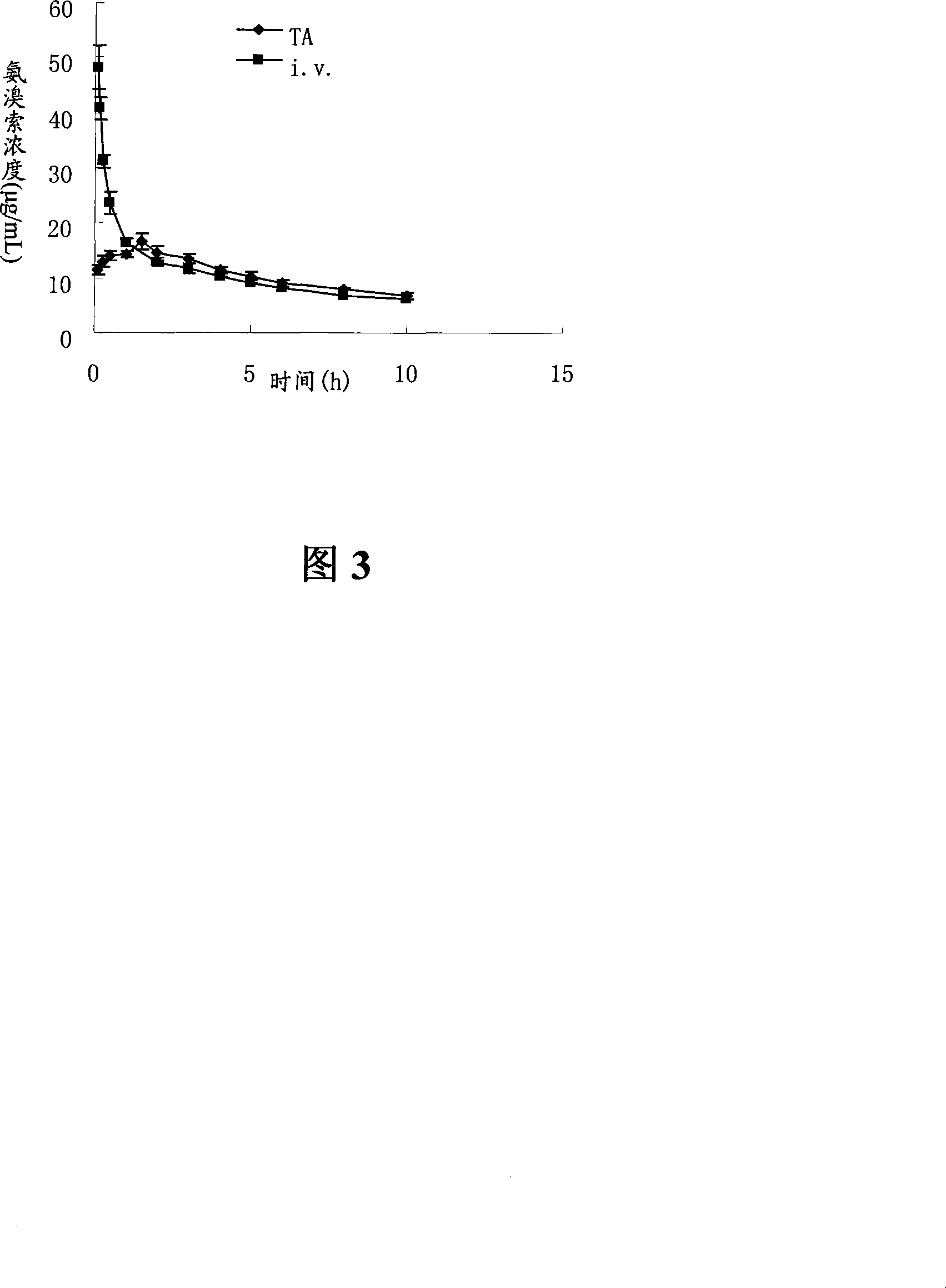
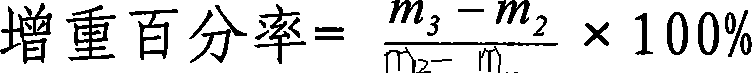Ambroxol hydrochloride dry powder inhalant and preparation thereof
A technology of ambroxol hydrochloride dry powder and ambroxol hydrochloride, which is applied in the field of medicine, can solve problems such as air pollution, unfavorable therapeutic effects, and large side effects, and achieve high-efficiency therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The present embodiment adopts the SD-1000 spray dryer of Japan EYELA Company.
[0022] Mix 2.5g (89.3%) of ambroxol hydrochloride and 0.3g (10.7%) of leucine and dissolve in 100mL of distilled water, adjust the pH to 6.0 with 0.1mol / L sodium hydroxide, and filter through a 0.2μm microporous membrane Afterwards, spray drying is carried out, and the process parameters are adopted: the inlet temperature is 110°C, and the spray speed is 1.8mL min -1 , pump pressure 170KPa, air flow 0.7m 3 min -1 Prepare the ambroxol hydrochloride dry powder inhaler, and fill the spray-dried powder in No. 3 capsules.
Embodiment 2
[0024] The present embodiment adopts the SD-1000 spray dryer of Japan EYELA Company.
[0025] Mix 2.5g (83.3%) of ambroxol hydrochloride and 0.5g (16.7%) of leucine and dissolve in 100mL of distilled water, adjust the pH to 6.0 with 0.1mol / L sodium hydroxide, and filter through a 0.2μm microporous membrane Afterwards, spray drying is carried out, and the process parameters are adopted: the inlet temperature is 110°C, and the spray speed is 1.8mL min -1 , pump pressure 170KPa, air flow 0.7m 3 min -1 Prepare the ambroxol hydrochloride dry powder inhaler, and fill the spray-dried powder in No. 3 capsules.
Embodiment 3
[0027] The present embodiment adopts the SD-1000 spray dryer of Japan EYELA Company.
[0028] Mix 2.5g (71.4%) of ambroxol hydrochloride and 1g (28.6%) of leucine and dissolve in 100mL of distilled water, adjust the pH to 6.0 with 0.1mol / L sodium hydroxide, and filter through a 0.2μm microporous membrane Carry out spray drying, adopt process parameters inlet temperature is 110 ℃, spray speed 1.8mL min -1 , pump pressure 170KPa, air flow 0.7m 3 min -1 Prepare the ambroxol hydrochloride dry powder inhaler, and fill the spray-dried powder in No. 3 capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com