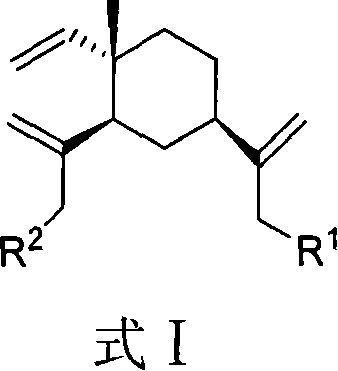
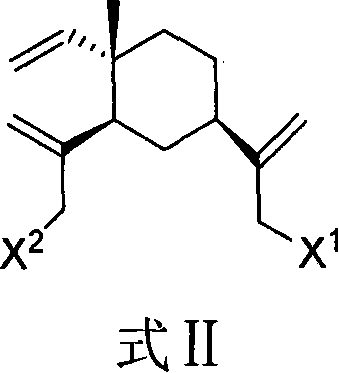
Beta-elemene amino acid derivatives as well as synthetic method and use thereof
A synthesis method and amino acid technology, which can be used in drug combinations, chemical instruments and methods, and medical preparations containing active ingredients, etc., can solve problems such as poor water solubility, limited clinical application, etc. efficacy, enhancing efficacy and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of β-elemene γ-butyric acid derivatives
[0029]
[0030] Add 0.1481g (1.4mmol) γ-butyric acid, 0.0609g NaOH, into 5mL CH 3 CH 2 OH and 1.6mL H 2 O, stirred at room temperature until completely dissolved, then added 0.64 g (2.7 mmol) of the chlorinated β-elemene mixture. Stir magnetically in an oil bath at 65°C, heat for 44 hours, the solution is transparent and blue, evaporate the solvent after the reaction is complete, and add CH 2 Cl 2 , use hydrochloric acid to adjust the pH value to 1~2, if no precipitation occurs, use CHCl 3 Extraction (10mL×3) gave a yellow oily liquid.
[0031] CH through a silica gel column 2 Cl 2 :CH 3 OH=10:1 (v / v) to separate the product, the developing agent is CH 2 Cl 2 :CH 3 When OH=5:1 (v / v), the Rf value of the product is 0.59.
[0032] Characterization: IR V max : 3080(C=C-H), 1709(C=O);
[0033] 1 H-NMR (CDCl 3 , TMS, 500MHz), δ0.99(s, 3H, CH 3 ), 1.20-1.79 (m, 6H), 1.70 (s, 3H, CH 3 ), 1.92-2....
Embodiment 2
[0035] Example 2 Synthesis of β-elemene β-alanine derivatives
[0036]
[0037] Add 0.1378g (1.5mmol) β-Ala, 0.1281g NaOH, 5mL CH 3 CH 2 OH and 1.8mL H 2 O, stirred at room temperature until completely dissolved, then added 0.70 g (3.0 mmol) of the chlorinated β-elemene mixture. Stir magnetically in an oil bath at 65°C, heat for 48 hours, the solution is transparent and yellow, evaporate the solvent after the reaction is complete, and add CH 2 Cl 2 , adjust the pH value to 1-2 with hydrochloric acid. If no precipitation occurs, use CHCl 3 Extraction (10mL×3) gave a yellow oily liquid.
[0038] CH through a silica gel column 2 Cl 2 :CH 3 OH=10:1 to separate the product, the developer is CH 2 Cl 2 :CH 3 When OH=5:1, the Rf value of the product is 0.59.
[0039] Characterization: IR V max : 3080(C=C-H), 1719(C=O), 1637(C=C);
[0040] 1 H-NMR (CDCl 3 , TMS, 500MHz), δ0.99(s, 3 H, CH 3 ), 1.20-1.35(m, 1H), 1.36-1.78(m, 5H), 1.73(s, 3H, CH 3 ), 1.99-2.19(m, 2H)...
Embodiment 3
[0043] Example 3 Synthesis of β-elemene L-alanine derivatives
[0044]
[0045] Add 0.7543g (8.4mmol) α-alanine, 0.5774g NaOH, 15mL CH 3 CH 2 OH and 5mL H 2 O, stirred at room temperature until completely dissolved, then added 4 g (16.8 mmol) of the chlorinated β-elemene mixture. Stir magnetically in an oil bath at 66°C, heat for 28 hours, the solution is transparent and yellow, evaporate the solvent after the reaction is complete, and add CH 2 Cl 2 , adjust the pH value to 1-2 with hydrochloric acid. If insoluble matter appeared, the solution was filtered to obtain a white solid with a yield of 57.4%.
[0046] Characterization: IR V max : 3080(C=C-H), 1637(C=O);
[0047] 1 H-NMR (CD 3 OD, TMS, 500MHz), δ0.99(s, 3H), 1.39-1.58(m, 6H), 1.59(d, J=6.07, 3H), 1.70(s, 3H), 2.01-2.10(m, 2H ), 3.59-3.63(m, 1H), 3.74-3.83(m, 2H), 4.57(s, 1H), 4.82(s, 1H), 4.89(s, 1H), 4.91(d, J=3.34, 1H ), 5.21 (s, 1H), 5.28 (s, 1H), 5.80 (dd, J=17.94, 10.9, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com