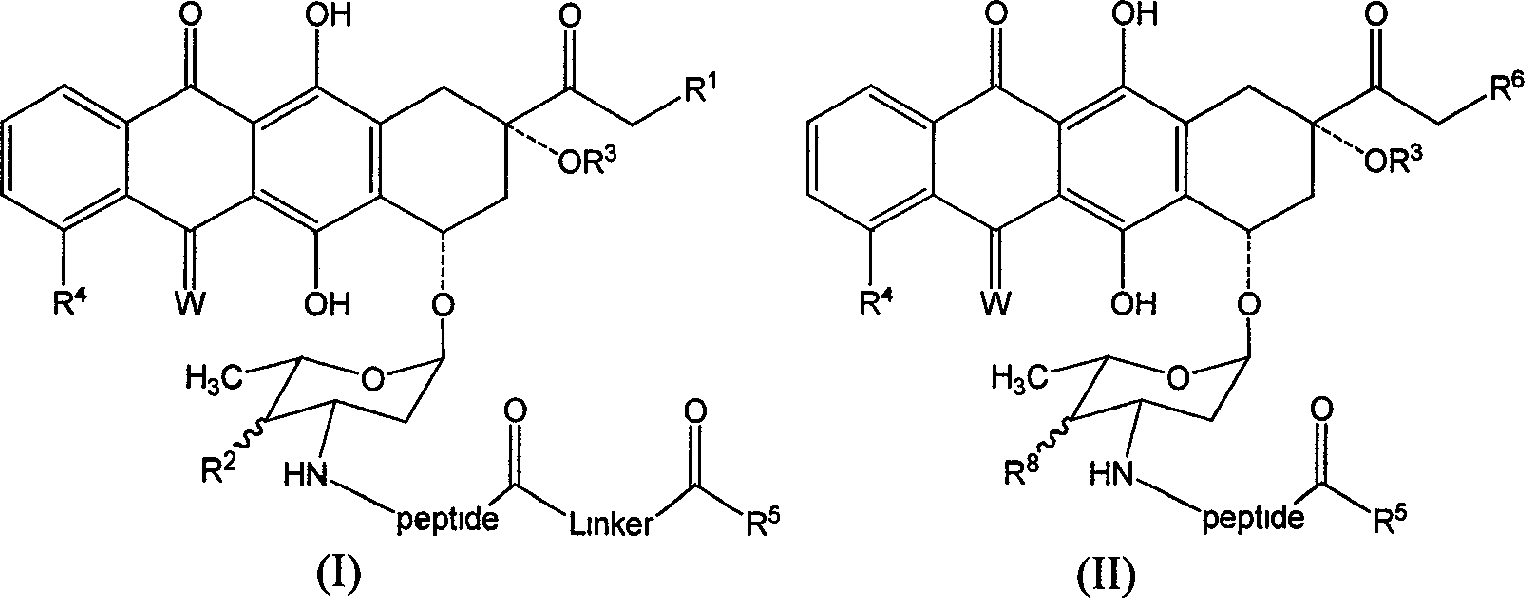
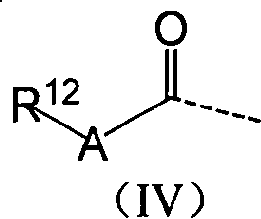
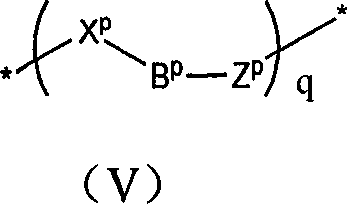Anthraquinones tetracyclic compound having anticancer activity
A technology of compounds and quinones, applied in the application field of active ingredients of anticancer drugs, can solve problems such as poor water solubility and difficulty in injection preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Boc-Diethylene glycol amine
[0146] Add 52.5 g (500 mmol) of diglycolamine and 200 ml of chloroform into a 1000 ml three-necked flask equipped with mechanical stirring, and cool down to 20° C. in an ice bath. 109g (500mmol) (Boc) 2 O was dissolved in 200ml of chloroform, added dropwise into the reaction flask with stirring, and stirred overnight at room temperature after dropping. After the reaction was completed, 400ml of water was added to the reaction solution, and the liquid was separated. The obtained organic phase was washed twice with water and twice with saturated brine, dried over anhydrous magnesium sulfate, filtered, spin-dried, and vacuum-dried to obtain 99.607g of compound I.
Embodiment 2
[0148] Boc-diethylene glycol amine p-toluenesulfonate
[0149] Add 99.607g of compound 1 and 102.0g of p-toluenesulfonyl chloride into a 500ml three-neck flask equipped with mechanical stirring, cool down to 15°C in an ice bath, drop 160ml of 20% NaOH aqueous solution, and stir overnight at room temperature. After the reaction was completed, the reaction solution was extracted three times with ethyl acetate, the organic phases were combined, washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered and spin-dried to obtain 150.258 g of compound 2.
Embodiment 3
[0151] tert-Butoxycarbonyl-aminoethoxyethylphthalimide
[0152] 87.689g (244.2mmol) of compound 2, 67.766g (366.3mmol) of phthalimide potassium salt, and 150ml of anhydrous DMF were added to a 250ml single-necked flask, stirred at room temperature for 1 hr, and then stirred overnight at 55°C. After the reaction was completed, 2000ml of water was added to the reaction solution, extracted three times with ethyl acetate, the organic phases were combined, washed twice with water, once with 5% NaOH, twice with saturated saline, dried and filtered over anhydrous magnesium sulfate, and the solvent was spun off to obtain the product 62.192 g. 1 H NMR: δ7.831(m, 2H), δ7.702(m, 2H), δ4.915(s, 1H), δ4.099(t, 2H), δ3.866(t, 2H), δ3. 669(t, 2H), δ3.500(t, 2H), δ3.238(t, 2H), δ1.389(s, 9H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com