Method for producing hydrogen peroxide by anthraquinone process
A technology of hydrogen peroxide and anthraquinone method, applied in chemical instruments and methods, peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, etc., can solve the liquid phase axis diameter Problems such as uneven distribution, low utilization efficiency of the reactor, and large mass transfer resistance of gas-phase hydrogen can be achieved to achieve uniform distribution, increase reactor efficiency, and reduce energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
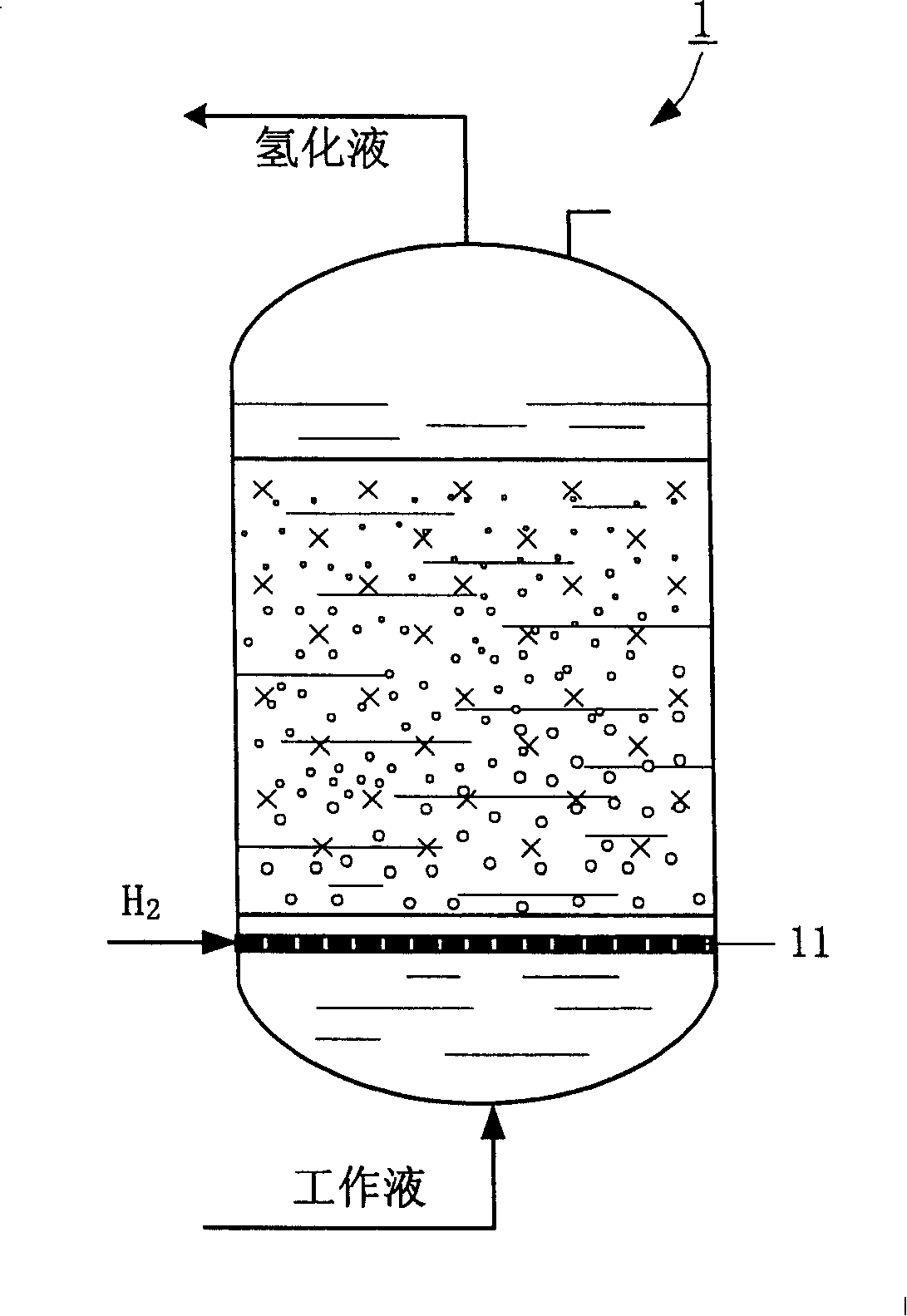
[0020] see figure 1 As shown, in this embodiment, a vertical fixed-bed reactor is adopted to carry out the catalytic hydrogenation reaction of anthraquinone, and the reactor is filled with 30ml Raschig annular Pd / Al 2 o 3 Catalyst and anthraquinone working liquid enter from the bottom of reactor 1, and the effective anthraquinone concentration of the working liquid is 130g / L. Hydrogen is also introduced from the lower part of the reactor, and is distributed into the working liquid in the form of bubbles through the hydrogen distributor 11. Control the hydrogenation reaction temperature in the reactor to about 50°C, the reaction pressure to 0.2MPa, and the liquid space velocity to 12h -1 , hydrogen air velocity 120h -1 .
[0021] After the hydrogenation reaction is completed, the hydrogenation working solution is discharged from the top of the reactor, and is transferred to the oxidation equipment (oxidation tower) to continue oxidation, and the oxidation product is separated ...
Embodiment 2
[0033] The fixed bed reactor and the operation process are the same as in Example 1, the hydrogenation reaction temperature is controlled at about 70°C, and the catalyst is spherical Pd / SiO 2 , the diameter is Φ2mm, and other evaluation conditions are the same as in Example 1.
[0034] Using the traditional feeding method, the hydrogen efficiency recorded is 3.58g / L; using the method of the present invention, using the lower feeding method, the hydrogen efficiency recorded is 5.50g / L, compared with the traditional feeding method, the hydrogen efficiency 53% increase.
Embodiment 3
[0036] The fixed-bed reactor and the operation process are the same as in Example 1, the hydrogenation reaction temperature is controlled at 60°C, the operating pressure is 0.4MPa, and the catalyst is Pd / Al 2 o 3 Φ2×5mm strips, other evaluation conditions are the same as in Example 1.
[0037] Using the traditional feeding method, the measured hydrogen effect is 2.55g / L; using the method of the present invention, using the lower feeding method, the recorded hydrogen effect is 5.11g / L, compared with the traditional feeding method, the hydrogen efficiency 100% increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com