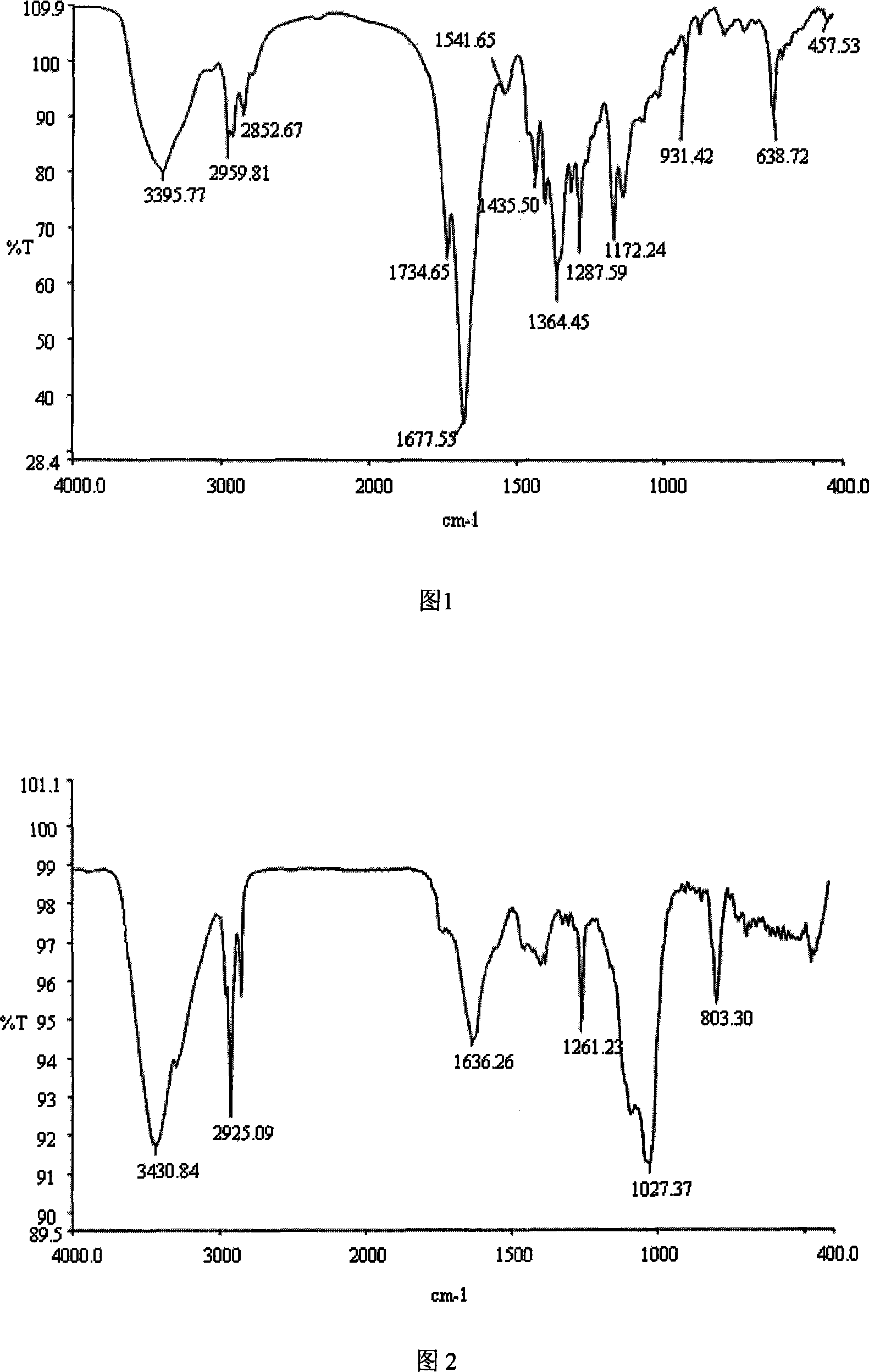
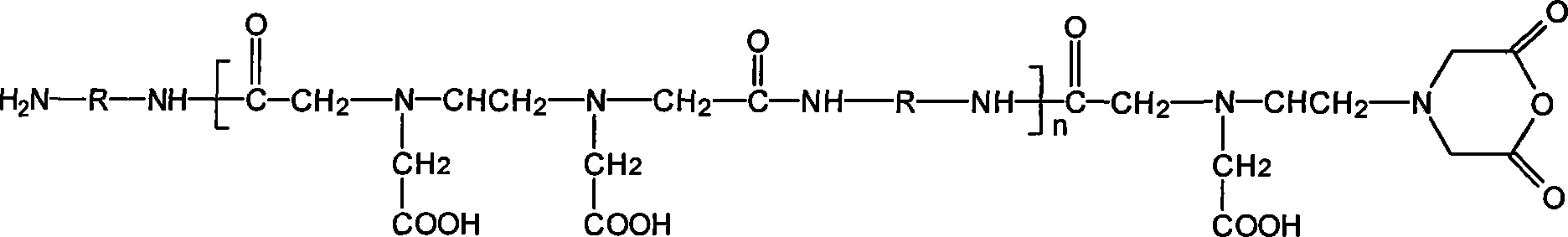
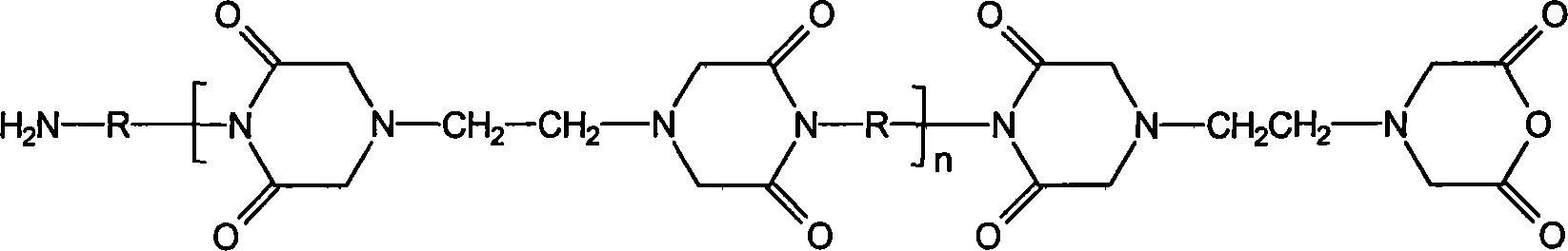
Hydrogel materials based on ethylenediaminetetraacetic dianhydride
A technology of ethylenediaminetetraacetic anhydride and hydrogel, which is applied to a class of hydrogel materials and uses based on ethylenediaminetetraacetic anhydride, can solve the problem of blood compatibility and histocompatibility and needs to be further verified, etc. problems, achieving good biocompatibility and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 EDTAh-BDA linear copolymer (pEDTAh-BDA) was prepared by reacting ethylenediaminetetraacetic anhydride (EDTAh) with butanediamine (BDA):
[0037] Add 1.5mol EDTAh and 15ml dimethyl sulfoxide (DMSO) into a three-necked flask equipped with a thermometer, a mechanical stirrer and a condenser. Under nitrogen protection, the temperature was slowly raised to 80° C., and 16 ml of 0.1 mol / ml DMSO solution of butanediamine was added dropwise with stirring, and the addition was completed within 90 minutes. After reacting for 6 hours, the reaction product was slowly dropped into tetrahydrofuran (THF) solution, and the precipitate was filtered. The precipitate was separated and purified three times using THF-DMSO system to obtain EDTAh-BDA linear copolymer (pEDTAh-BDA). Determination of Number Average Molecular Weight of pEDTA-BDA by Ninhydrin Chromogenic Method M ‾ n = 7900 , ...
Embodiment 2
[0038] Example 2 The imide-containing copolymer: EDTAh-BDA-Imide was prepared from the product pEDTAh-BDA in Example 1. Put 0.8 g of phosphoric acid and 100 g of pEDTAh-BDA into a two-necked flask, and slowly raise the temperature to 160° C. in an oil bath under the protection of nitrogen. After reacting for 5 hours, the reaction product was purified three times with DMSO-THF co-precipitation system to obtain pure EDTAh-BDA-Imide.
Embodiment 3
[0039] Example 3 The product EDTAh-BDA-Imide of Example 2 was used as a raw material, and butanediamine was used as a crosslinking agent to prepare the crosslinked product BDA-crosslinked-pEDTAh-BDA:
[0040] After dissolving 20 g of EDTAh-BDA-Imide (about 2.53 mmol) in 50 ml of dimethyl sulfoxide, slowly add 8 ml of 0.01 mol / ml butanediamine (80 mmol) in DMSO solution dropwise at a reaction temperature of 25°C. After the dropwise addition was completed, the reaction was carried out at 25° C. for 10 hours. The reaction liquid suspension was poured into 300ml of distilled water and left to stand for 14 days to obtain a large amount of swollen matter. Replace the distilled water, continue soaking the swollen product for 8 hours, repeat this operation twice, and dry in vacuum at room temperature to obtain the BDA-crosslinked cross-linked product BDA-crosslinked-pEDTAh-BDA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com