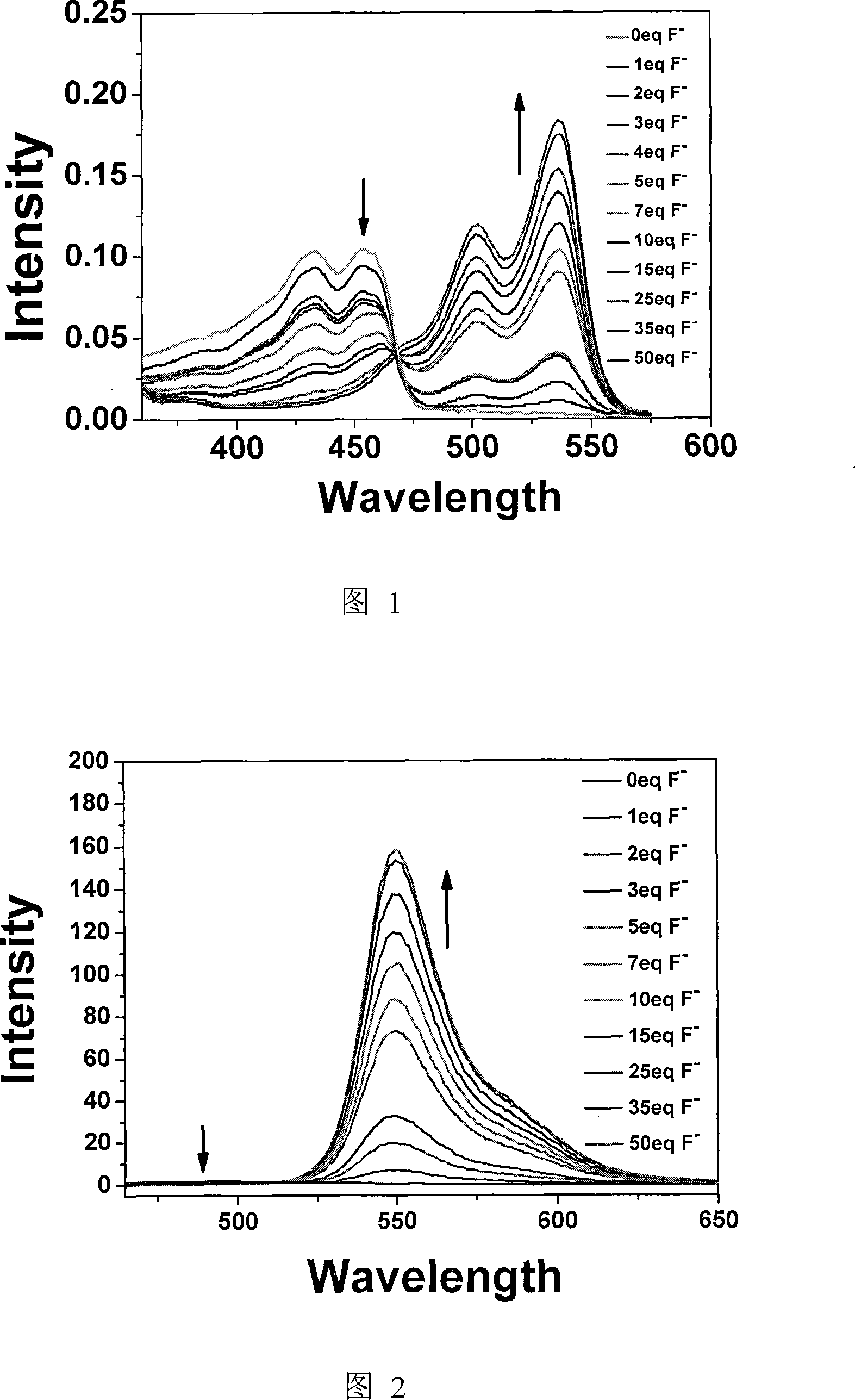
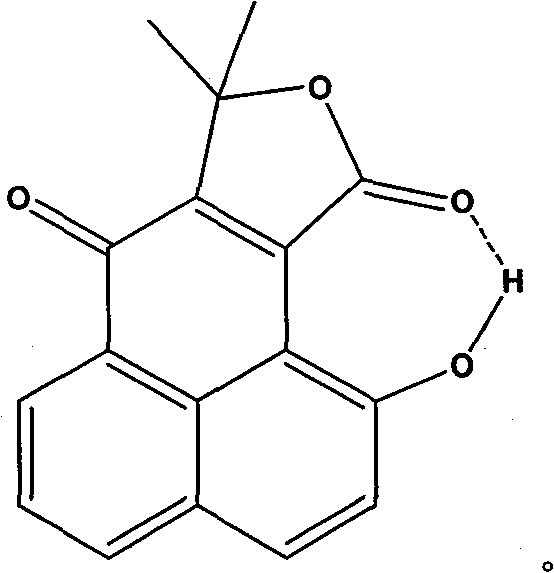
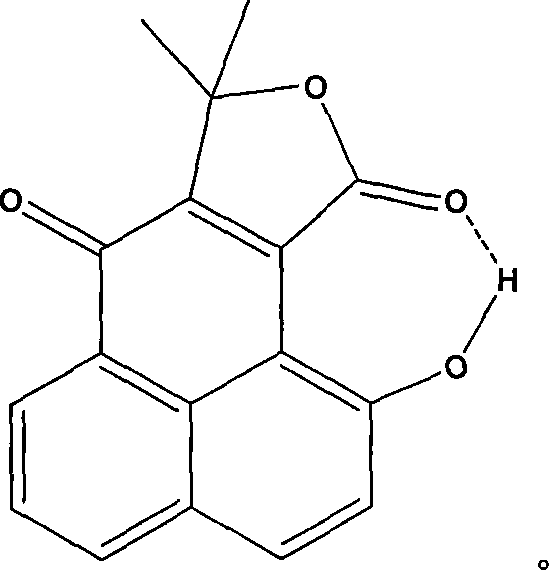
Fluorescent chemical sensor as well as preparation and detection method thereof
A chemical sensor and fluorescence technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of multiple reaction steps, complex post-reaction processing, expensive design and synthesis of fluorescent chemical sensors, etc., to achieve fast sensing, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Mix 1mmol of 1,8-diiodonaphthalene, 10mmol of 2-methyl-3-butyn-2-ol, 10% molar equivalent of cuprous iodide, and 5% molar equivalent of tetrakistriphenylphosphine palladium as catalyst in 35~ 50ml of diisopropylamine organic solvent was reacted at 23°C for 16h, and 1,8-bis(3-methyl-3-hydroxy-1-butynol)naphthalene was isolated after simple post-treatment with a yield of 99%. The reaction formula is as follows:
[0022]
[0023] Compound structural characteristics: mp(toluene)120-121℃; 1 H NMRδCDCl 3 (Bruker AVANCED MX500): 1.69 (s, 6H), 3.28 (br, 1H), 7.38 (t, 1H, J=5.2Hz), 7.69 (dd, 1H, J 1 =7.25Hz,J 2 =1.05Hz), 7.76(d, 1H, J=8.25Hz)ppm.
Embodiment 2
[0025] 1mmol 1,8-diiodonaphthalene, 1mmol 2-methyl-3-butyn-2-ol, 10% molar equivalent of triphenylphosphine, 10% molar equivalent of cuprous iodide, 5% molar equivalent of catalyst Tetrakistriphenylphosphine palladium was mixed in 35-50ml triethylamine organic solvent, reacted at 23°C for 10-13 hours, and was isolated by simple post-treatment to obtain 1-iodo-8-(3-methyl-3-hydroxy-1 -butynol)naphthalene, 86% yield. The reaction formula is as follows:
[0026]
[0027] Compound Structural Features: 1 H NMRδCDCl 3 (Bruker AVANCE DMX500): 8.26 (dd, J 1 =7.0Hz,J 2 =0.75Hz), 7.80(m, 3H), 7.39(t, 1H, J=7.25Hz), 7.08(t, 1H, J=7.75Hz), 2.10(br, 1H), 1.69(s, 6H)ppm .
Embodiment 3
[0029] With 1mmol 1,8-diiodonaphthalene, 10mmol 2-methyl-3-butyn-2-alcohol, 10% molar equivalent of triphenylphosphine, catalytic amount (0.05~0.10 molar equivalent) of cuprous iodide and Ditriphenylphosphine palladium dichloride is mixed in 75-100ml of triethylamine organic solvent, vacuumed and ventilated with nitrogen, then added with 3-5mmol of water, heated and refluxed for about 8-14 hours and separated to obtain the product.
[0030] The organic solvent of the present invention is trialkylamine; 3-5 molar equivalents of water must be added; the chromatographic column and eluent are silica gel chromatographic column and mixed eluent of dichloromethane and n-hexane. The reaction formula is as follows:
[0031]
[0032] Compound Structural Features: 1 H NMRδCDCl 3 (Bruker AVANCE DMX400): 1.80(s, 6H), 7.17(d, 1H, J=6.4Hz), 7.64(t, 1H, J=7.8Hz), 7.94(d, 1H, J=9.2Hz), 8.12 (dd, 1H, J 1 =7.6Hz,J 2 = 1.2Hz), 8.68(dd, 1H, J 1 =7.6Hz, J2=1.2Hz), 13.22(s, 1H)ppm; 13 C NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com