Spiral tube membrane and preparation and application thereof
A technology of tube membrane and spinal cord, applied in the fields of medicine, medical biomaterials and medical tissue engineering, can solve the problems of paraplegia that is difficult to repair, paraplegia is difficult to cure, and the effect is not good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
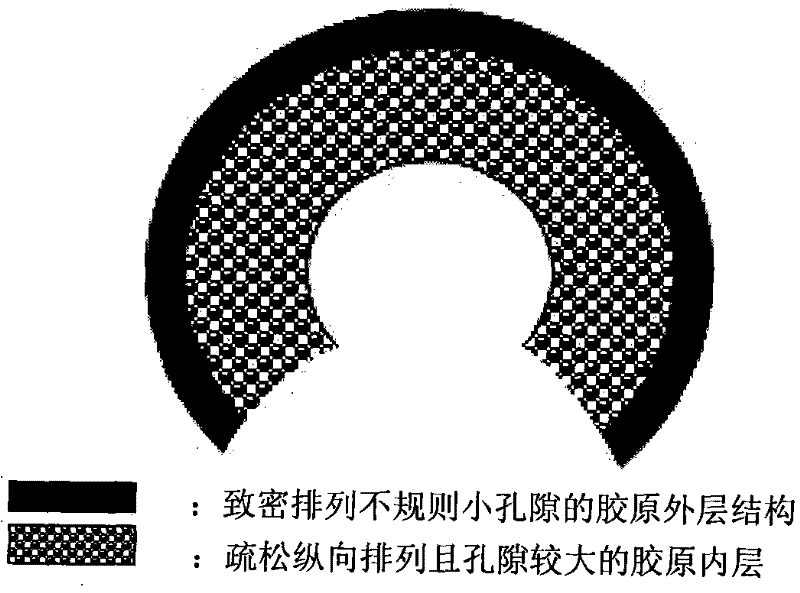
[0038]Make type I collagen into collagen suspension with a concentration of 0.5%, pour it into an open tubular mold, and freeze vertically at a speed of 1 mm / min at -80 degrees Celsius to form a composite that meets the requirements. The loose collagen inner layer of the collagen tubule;
[0039] Make type I collagen into a 10% collagen suspension, inject it into the above-mentioned open tubular mold with loose collagen, and freeze it vertically at -80 degrees Celsius at a slow speed of 1 mm / min. Sub-freezing to form a dense collagen outer layer that meets the requirements of the composite collagen tube membrane
[0040] The prepared loose collagen inner layer and dense collagen outer layer are compounded and conventionally freeze-dried to make a composite collagen tube membrane;
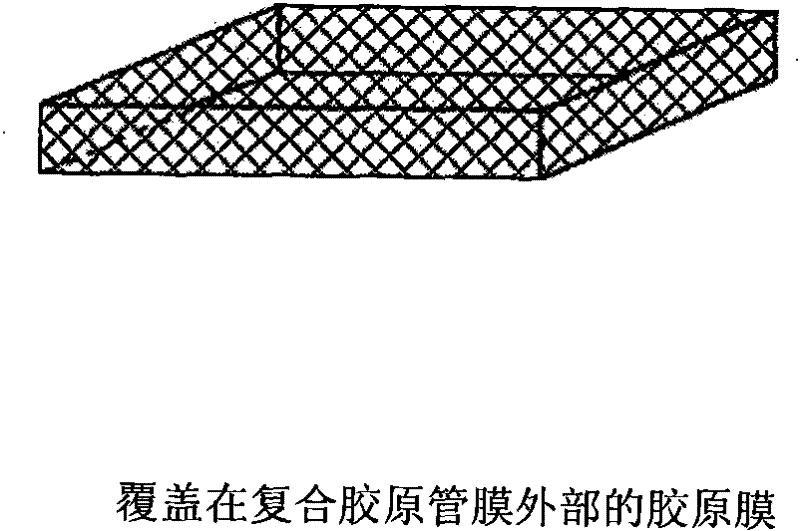
[0041] The type I collagen is made into a collagen suspension with a concentration of 5%, injected into a conventional freeze-drying apparatus, and frozen routinely to form a sheet-like collagen fi...
Embodiment 2
[0043] Make type I collagen into a 10% collagen suspension, pour it into an open tubular mold, and freeze vertically at a slow speed of 1 mm / min at -100 degrees Celsius to form a composite that meets the requirements. The loose collagen inner layer of the collagen tubule;
[0044] Make type I collagen into a collagen suspension with a concentration of 0.5%, inject it into the above-mentioned open tubular mold with loose collagen, and freeze it vertically at -100 degrees Celsius at a slow speed of 2 mm / min. Sub-freezing to form a dense collagen outer layer that meets the requirements of the composite collagen tube membrane
[0045] The prepared loose collagen inner layer and dense collagen outer layer are compounded and conventionally freeze-dried to make a composite collagen tube membrane;
[0046] The type I collagen is made into a collagen suspension with a concentration of 1%, injected into a conventional freeze-drying apparatus, and routinely frozen to form a sheet-like c...
Embodiment 3
[0048] Make type I collagen into a 5% collagen suspension, pour it into an open tubular mold, and freeze it vertically at a rate of 1 mm / min at -90 degrees Celsius to form a composite that meets the requirements. The loose collagenous inner layer of the collagen tubule.
[0049] Make type I collagen into a collagen suspension with a concentration of 5%, inject it into the above-mentioned open tubular mold that retains loose collagen, freeze vertically at a speed of 2 mm / min at -90 degrees Celsius, and Freeze twice to make it form a dense collagen outer layer that meets the requirements of the composite collagen tube membrane.
[0050] The prepared loose collagen inner layer and dense collagen outer layer are combined and freeze-dried to make a composite collagen tube membrane.
[0051] The type I collagen is made into a collagen suspension with a concentration of 0.5%, injected into a conventional freeze-drying apparatus, and conventionally frozen to form a sheet-like collage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com