Method for synthesizing penem-like pharmaceutical intermediate 4AA
A synthesis method and 4AA technology, which are applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, and production of bulk chemicals, etc., can solve the problems of large dosage, difficult control, heavy metal pollution, etc. High, mild reaction conditions, less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
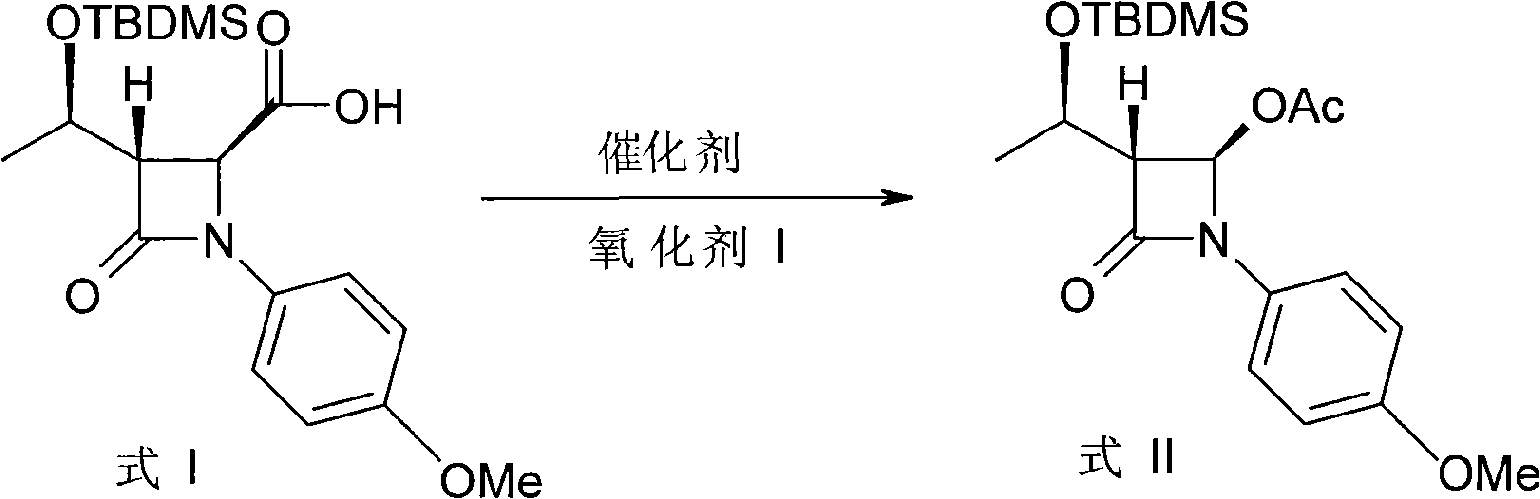
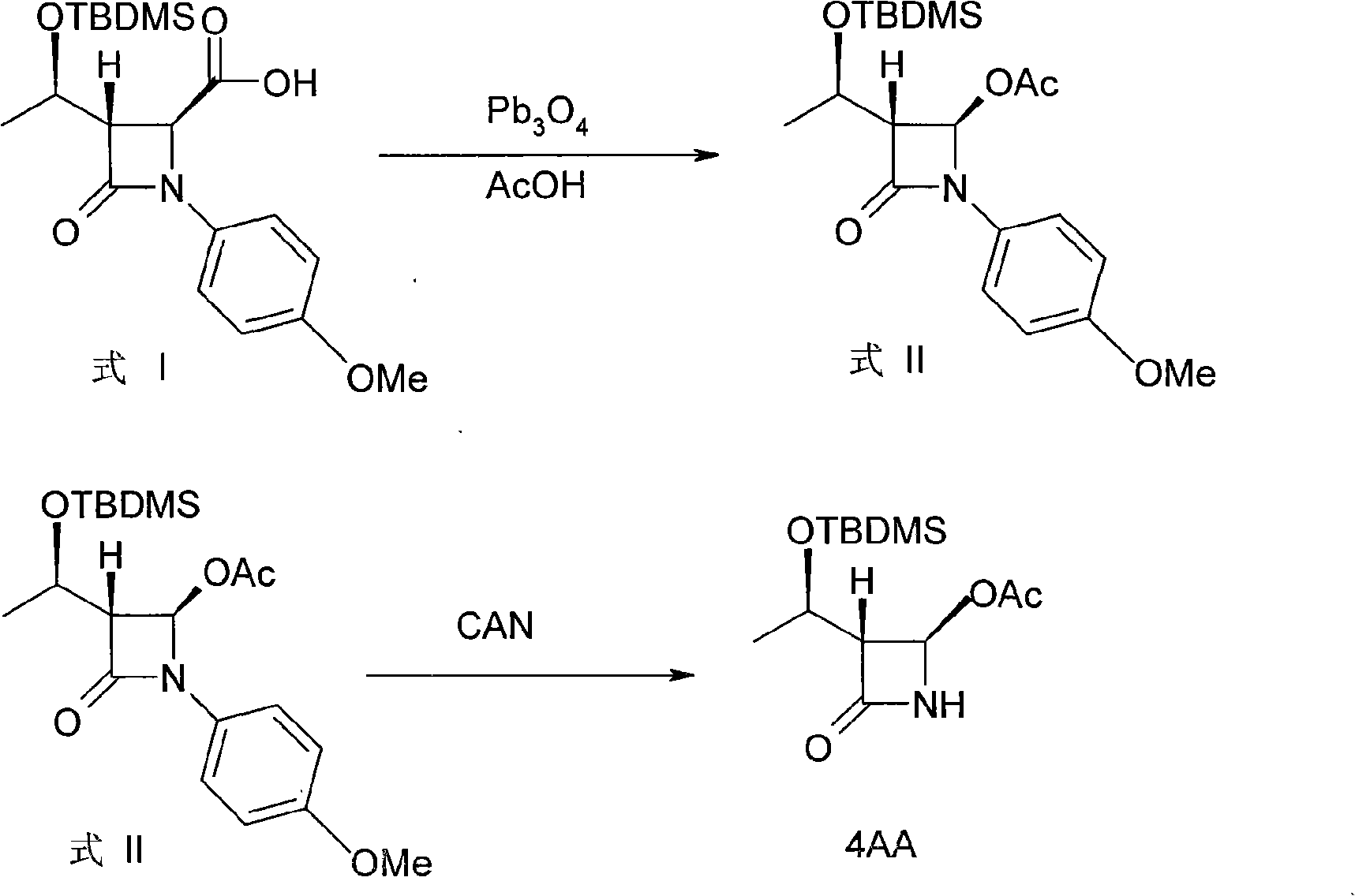
[0026] Example 1: (3R, 4R)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-p-methoxyphenyl-2-aza Synthesis of cyclobutanone (formula II)
[0027] Add 250 mL of glacial acetic acid and a small amount of acetic anhydride into a three-necked flask, and put in (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-carboxy-1-p-methoxy phenyl-2-azetidinone (formula I) 10g and KHSO 5 80g, stir and heat up to about 40°C, and add 0.5g of porphyrin manganese catalyst in batches within 30min. Then keep warm at 45-50°C for 8 hours, filter after the reaction is completed, wash the filter cake with water and alcohol in turn, dry, recover the catalyst and apply it mechanically, the filtrate is distilled under reduced pressure to recover the solvent, the solid is dissolved in dichloromethane, and water, saturated NaHCO 3 , washed with saturated brine, the oil layer was dried, and the solvent was evaporated to dryness, and the product was obtained after recrystallization, which was detected ...
Embodiment 2
[0028] Example 2: (3R, 4R)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-p-methoxyphenyl-2-aza Synthesis of cyclobutanone (formula II)
[0029] Add 250 mL of glacial acetic acid and a small amount of acetic anhydride into a three-necked flask, and put in (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-carboxy-1-p-methoxy Base phenyl-2-azetidinone (formula I) 10g, stir and heat up to about 40°C, pass into O 2 , Add 0.5 g of porphyrin manganese catalyst in batches within 1 h. Then keep warm at 45-50°C for 8 hours, filter after the reaction is completed, wash the filter cake with water and alcohol in turn, dry, recover the catalyst and apply it mechanically, the filtrate is distilled under reduced pressure to recover the solvent, the solid is dissolved in dichloromethane, and water, saturated NaHCO 3 , washed with saturated brine, the oil layer was dried, and the solvent was evaporated to dryness, and the product was obtained by recrystallization, which was detected as...
Embodiment 3
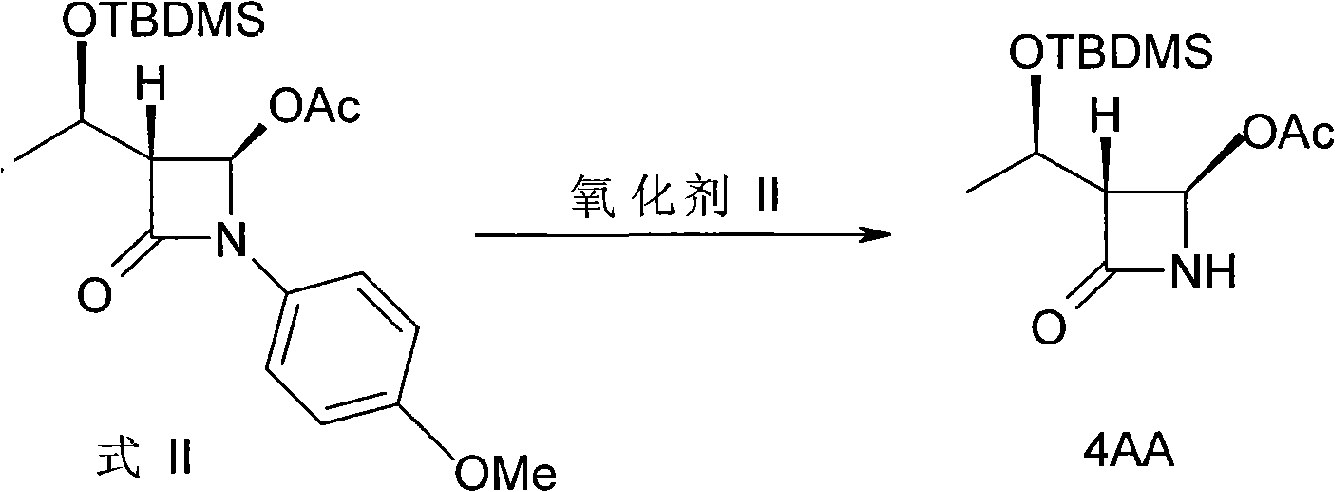
[0030] Example 3: Synthesis of (3R, 4R)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetoxy-2-azetidinone (4AA)
[0031] (3R, 4R)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-p-methoxyphenyl-2-azetidinone (Formula II) 13g, dissolved in 250mL of methanol, cooled to 0 ° C, passed into O 3 React for 3h, after the reaction is completed, add Na 2 S2 o 3 And thiourea, stirred at room temperature for 30min. The reaction solution was concentrated to 1 / 3. The concentrated solution was cooled to -10-0°C, white crystals were precipitated, filtered, dried, and recrystallized in n-hexane to obtain white crystals, which were detected as 4AA with a yield of 90%. (mp: 107-108°C, [α] D 23 : 49.08 (c1.0, CHCl 3 ); Literature: mp: 106-108°C, [α] D 23 : 48.75 (c1.05, CHCl 3 ))
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com