BaCoO3 based perovskite type ceramic oxygen-permeable membrane material with Sn, Fe doped at B position
A perovskite-type, oxygen-permeable membrane technology, applied in semi-permeable membrane separation, membrane technology, oxygen production, etc., can solve the problems of poor stability of oxygen-permeable membrane materials, and achieve the effect of good phase structure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
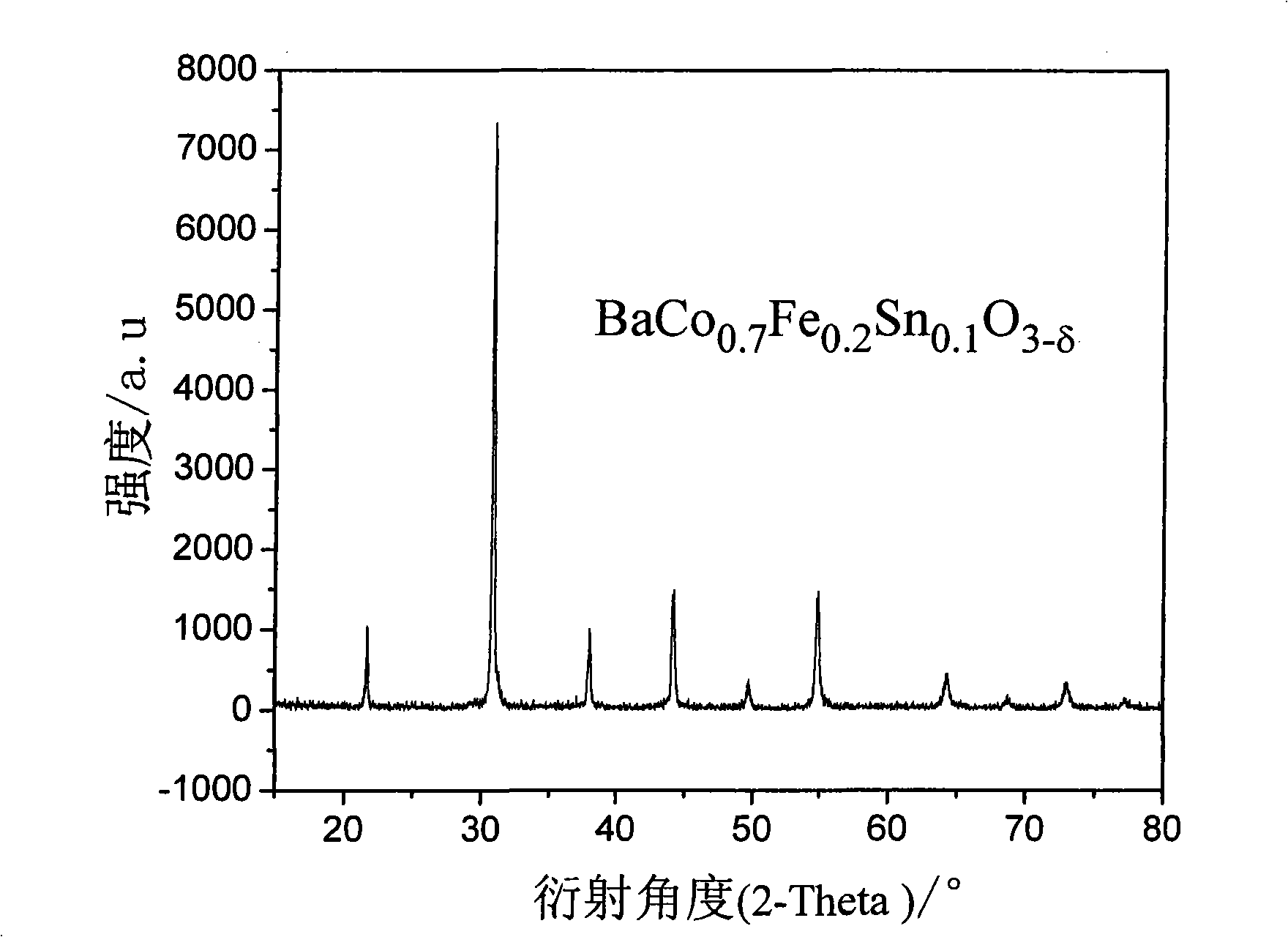
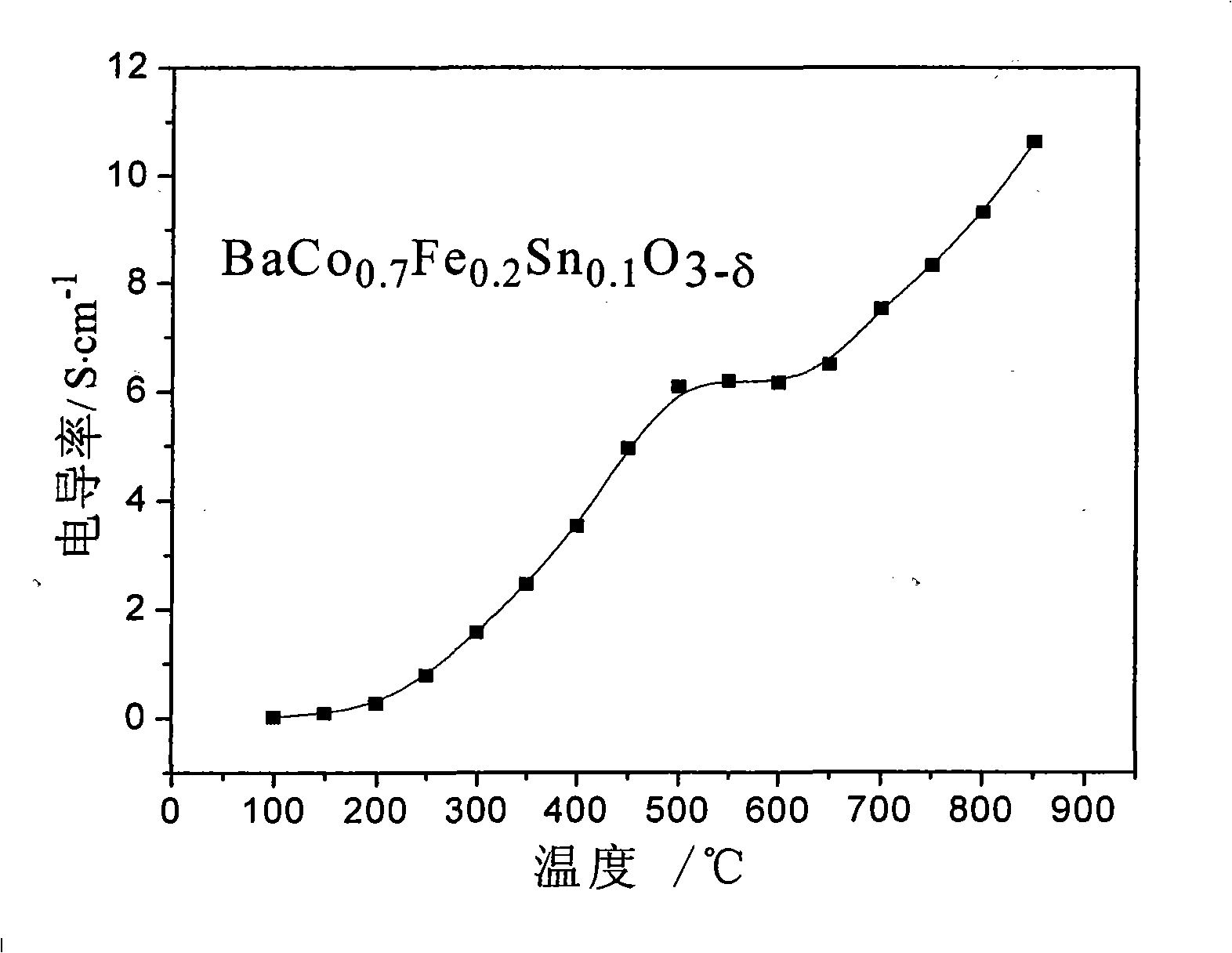
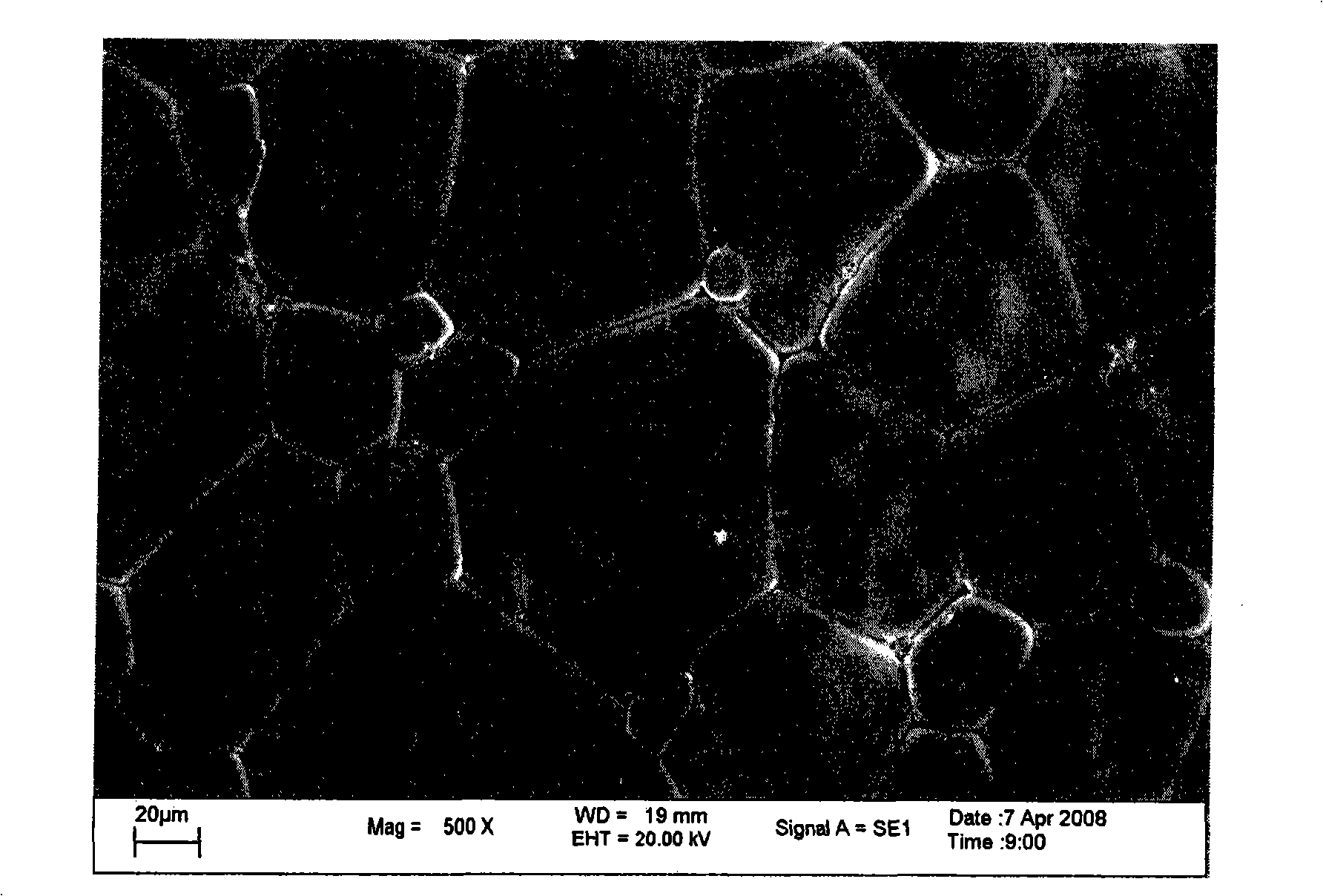
[0017] Example 1: BaCo 0.7 Fe 0.2 sn 0.1 o 3-δ Synthesis by solid phase reaction
[0018] With 29.601g of BaCO 3 (analytical pure), 2.261g of SnO 2 (analytical pure), 2.396g of Fe 2 o 3 (analytical pure), 26.154g of CoCH 3 COOH (analytical pure) is the raw material, that is, according to BaCo 0.7 Fe 0.2 sn 0.1 o 3-δ The mixture was prepared in the ratio of the elements, using alcohol as the medium, milled in an agate ball mill jar for 6 hours, mixed evenly, and dried in an oven. After the dried material was calcined at 1000°C for 10 hours, it was ball milled again with agate balls as the grinding medium for 4 hours, and then put into a drying oven for drying. After the drying is complete, grind it evenly in a mortar, add 1.0wt.% PVA to mix and dry it, and then dry press it in a steel casting mold. The pressed sample strip was heated to 1180°C in a high-temperature furnace, and after 10 hours of heat preservation, a dense BaCo 0.7 Fe 0.2 sn 0.1 o 3-δ sample. T...
Embodiment 2
[0019] Example 2: BaCo 0.7 Fe 0.22 sn 0.08 o 3-δ Synthesis by solid phase reaction
[0020] With 29.601g of BaCO 3 (analytical pure), 1.808g of SnO 2 (analytical pure), 2.635g of Fe 2 o 3 (analytical pure), 26.154g of CoCH 3 COOH (analytical pure) is the raw material, that is, according to BaCo 0.7 Fe 0.22 sn 0.08 o 3-δ The mixture was prepared in the ratio of the elements, using alcohol as the medium, milled in an agate ball mill jar for 6 hours, mixed evenly, and dried in an oven. After the dried material was calcined at 1000°C for 10 hours, it was ball milled again with agate balls as the grinding medium for 4 hours, and then put into a drying oven for drying. After the drying is complete, grind it evenly in a mortar, add 1.0wt.% PVA to mix and dry it, and then dry press it in a steel casting mold. The pressed sample strip was heated to 1180°C in a high-temperature furnace, and after 10 hours of heat preservation, a dense BaCo 0.7 Fe 0.22 sn 0.08 o 3-δ samp...
Embodiment 3
[0021] Example 3: BaCo 0.7 Fe 0.18 sn 0.12 o 3-δ solid phase reaction method
[0022] With 29.601g of BaCO 3 (analytical pure), 2.713g of SnO 2 (analytical pure), 2.156g of Fe 2 o 3 (analytical pure), 26.154g of CoCH 3 COOH (analytical pure) is the raw material, that is, according to BaCo 0.7 Fe 0.18 sn 0.12 o 3-δ The mixture was prepared in the ratio of the elements, using alcohol as the medium, milled in an agate ball mill jar for 6 hours, mixed evenly, and dried in an oven. After the dried material was calcined at 1000°C for 10 hours, it was ground into powder with an agate mortar, and then ball milled with agate balls as the grinding medium for 4 hours, and then put into a drying oven for drying. After the drying is complete, grind it evenly in a mortar, add 1.0wt.% PVA to mix and dry it, and then dry press it in a steel casting mold. The pressed sample strip was heated to 1180°C in a high-temperature furnace, and after 10 hours of heat preservation, a dense B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com