Single-chain fragment antibody-polypeptide amalgamation protein and uses thereof
A technology of fusion proteins and fragments, applied in the fields of biology and medicine, can solve the problems of weak gene expression effect and cannot meet clinical application, etc., and achieve the effect of improving expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Example 1. Construction of a plasmid with a fusion protein that binds to Her2 receptor and carries a bidirectional function of siRNA drug. After finding the full-length sequence of the Her2 single-chain fragment antibody from the gene library, use primer software to design relevant primers.
[0039] Designed Her2-ScFv primers:
[0040] Forward primer: NcoI is an endonuclease
[0041] 5'-GGGCCATGGCCCAGGTGCAGCTGTTGCAGTCTGGGGCAGAG-3'
[0042] Anti-primer: NotI is an endonuclease
[0043] 5'-TTGCGGCCGCTCCGGAATTCACCTAGGACGGTCAGCTTGGTCCC-3'
[0044] Designed protidine peptide primers:
[0045] Positive primer: NotI is an endonuclease
[0046] 5'-CCGGAGCGGGCCGCAATGGCCAGGTACAGATGCTG-3'
[0047] Anti-primer: PpuMI as endonuclease, stop codon and 6 histidines
[0048] 5'-GCCGGGTCCCAGGAAAGGATCAGATCTGCATTAATGGTGGTGGTGATGATGAGATCTGTGTCTTTCTACATCTCGGTCTG-3'
[0049] Clone relevant target fragments by PCR: Her2-ScFv PCR reaction system 1:50ul system:
[0050] wxya 2 O 37ul
...
Embodiment 2
[0100] Example 2 Small amount extraction of pACgp67B-Her2-scfv-protamine plasmid
[0101]Collect the bacteria by centrifugation at 12000g×1min. (Generally, 3-4ml bacterial solution is used for extracting a portion), discard the supernatant. Add 250ul ice-bath Buffer S1 (containing RNaseA), vortex and oscillate to fully suspend the bacteria. Add 250ul Buffer S2 and mix gently 6 times. (This process does not exceed 5 minutes) Add 350ul Buffer S3, and mix gently 6 times. Centrifuge at 12000g x 10min. Take the supernatant, pass through the DNA preparation tube at 12000g×1min, and discard the filtrate. Add 700ul BufferW1 to the DNA preparation tube, 12000g×1min, discard the filtrate. Add 500ul Buffer W2 to the DNA preparation tube, 12000g×1min, discard the filtrate. Centrifuge again at 12000 g x 1 min. Add 40ul of ddH2O (or EB buffer) to the center of the membrane of the DNA preparation tube, and let stand at room temperature for 1min (preheating the EB buffer at 50°C may ha...
Embodiment 3
[0102] Example 3. Plasmid pACgp67B-Her2-scfv-protamine constructed by enzyme digestion and identification of Her2 single-chain fragment antibody and protamine polypeptide
[0103] Perform enzyme digestion to identify the pACgp67B-Her2-scfv-protamine plasmid fragment:
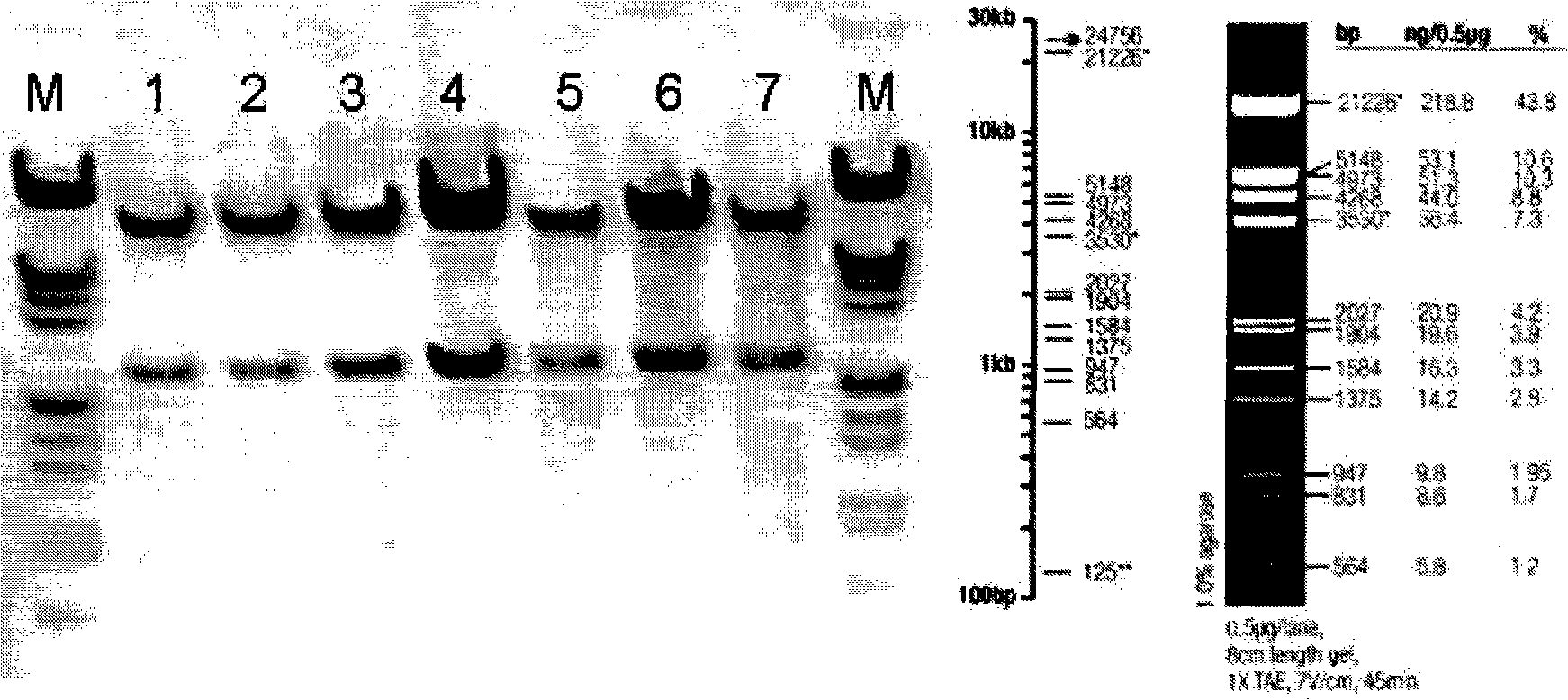
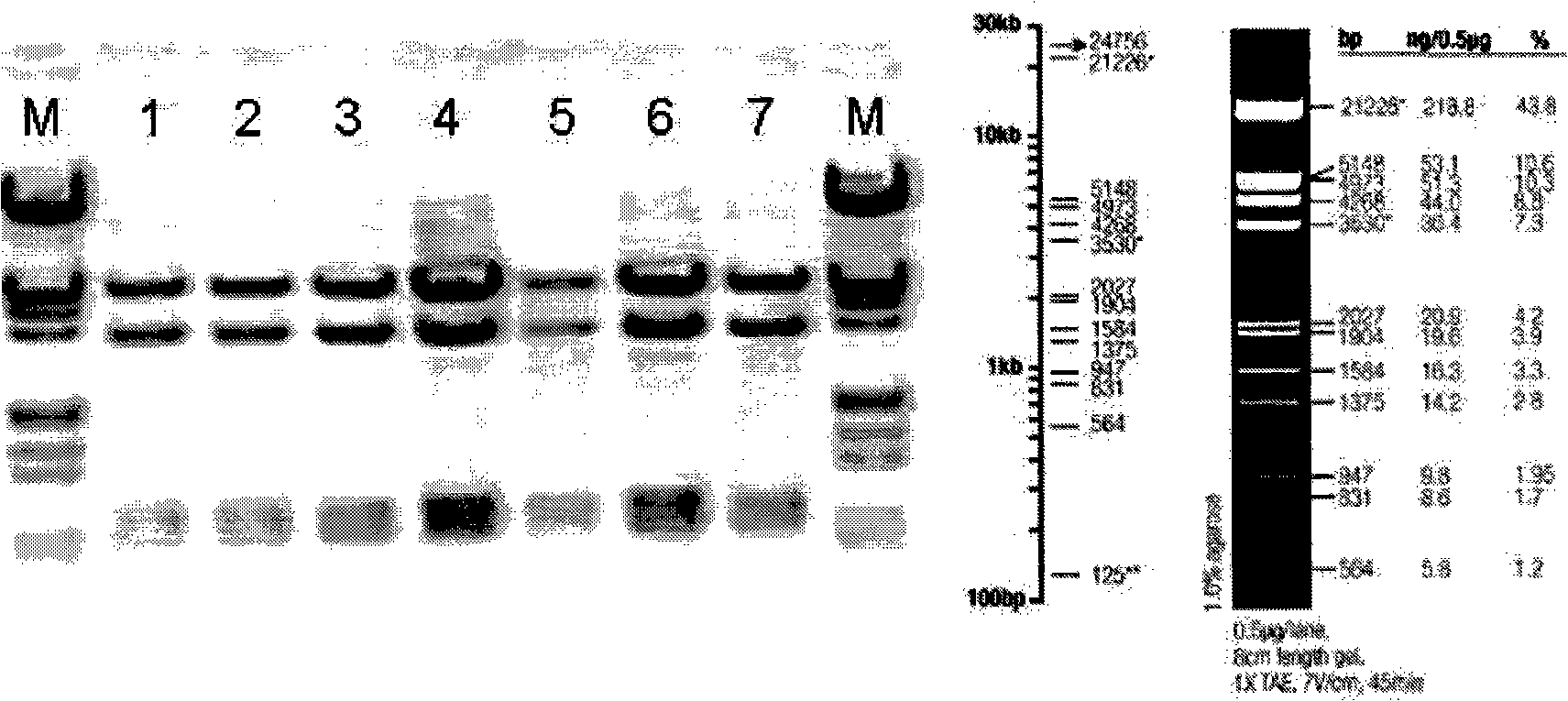
[0104] ①. The restriction site of BamHI (G^GATCC) is at 4259; the restriction site of XhoI (C^TCGAG) is at 1902; therefore, the size of the digested band is 2357bp, and the position is accurate. Such as figure 1 As shown, pACgp67B-Her2-scfv-protamine was identified by digestion with BamHI and XhoI. M is a marker, 1, 2, 3, 4, 5, 6, and 7 are amplified and extracted plasmid DNA from different clones. After enzyme digestion, there is a corresponding band above 2027bp, which is consistent with the position of the expected band.
[0105] ②. The enzyme cutting sites of HindIII (A^AGCTT) are at: 2, 5328, 6256, and 7292, so the sizes of the four bands after enzyme cutting are: 5326bp, 3423bp, 1036bp, 928bp, and the po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com