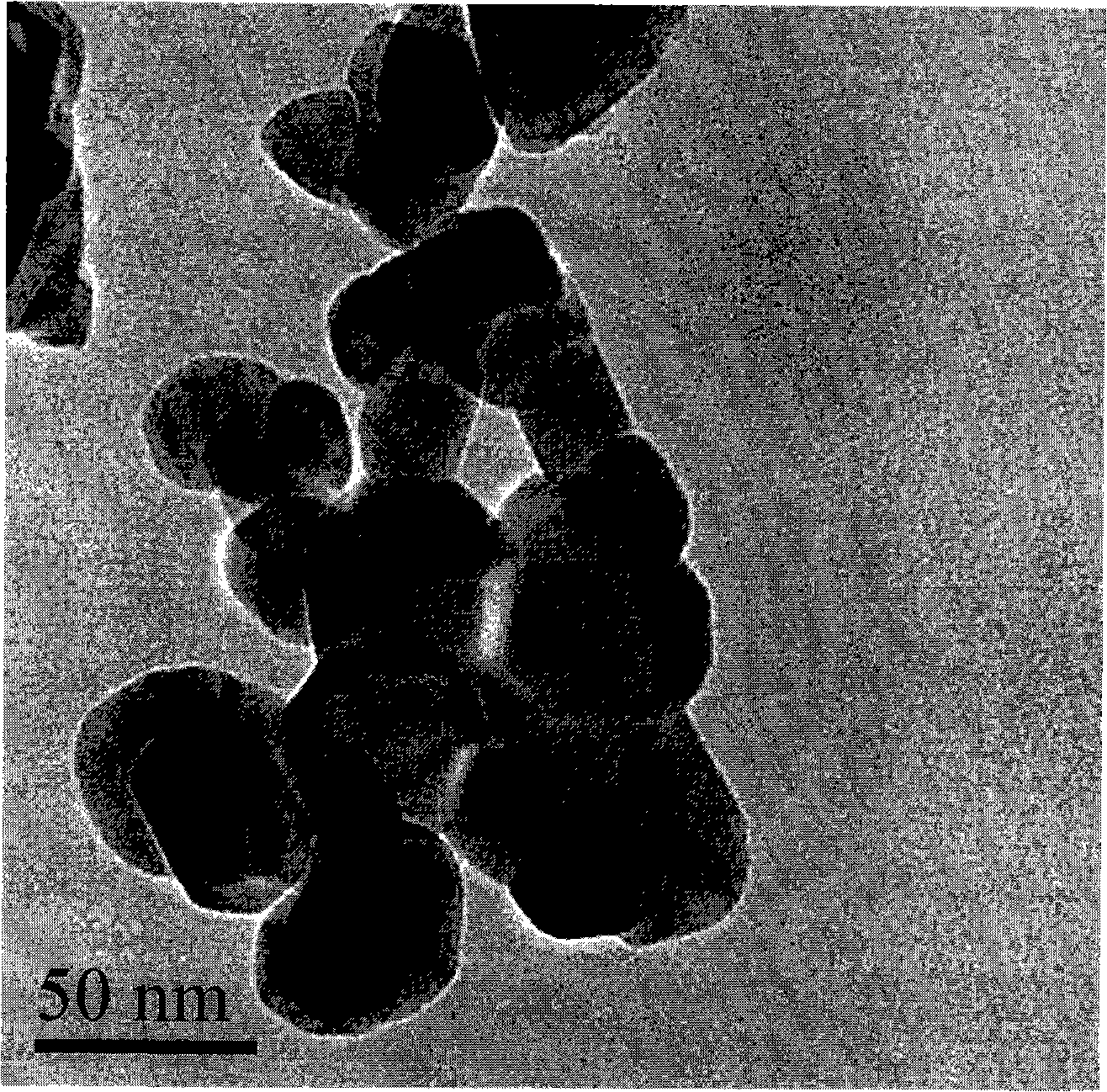Nano-scaled materials ZnMn2O4 for lithium storage and preparation thereof
A lithium storage and nano technology, applied in manganate/permanganate and other directions, can solve the problems of high cost and cobalt toxicity, and achieve the effects of low cost, high specific capacity and stable charge-discharge cycle performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 18.9 grams of zinc nitrate and 35.8 grams of manganese nitrate were weighed, added to 43.2 grams of acrylic acid, and a homogeneous solution was formed by magnetic stirring, wherein the molar ratio of zinc nitrate, manganese nitrate and acrylic acid was 1:2:6. 0.1 ml of 5% ammonium persulfate solution was added to the above solution as an initiator, and after mixing uniformly, it was allowed to stand at 80° C. for 2 hours to make the polymerization reaction fully proceed to generate a polyacrylate precursor. The polyacrylate precursor was then dried at 120°C for 24 hours, followed by calcination at 600°C for 6 hours to obtain a brown powder product, ZnMn 2 O 4 .
Embodiment 2
[0024] 13.6 grams of zinc chloride and 34.6 grams of manganese acetate were weighed, added to 72 grams of acrylic acid, and a homogeneous solution was formed by magnetic stirring, wherein the molar ratio of zinc chloride, manganese acetate and acrylic acid was 1:2:10. 0.5 ml of 5% ammonium persulfate solution was added to the above solution as an initiator, and after mixing uniformly, it was allowed to stand at 80° C. for 2 hours to make the polymerization reaction fully proceed to form a polyacrylate precursor. The polyacrylate precursor was then dried at 120°C for 24 hours, followed by calcination at 700°C for 6 hours to obtain ZnMn as a brown powder product 2 O 4 .
Embodiment 3
[0026] 28.7 grams of zinc sulfate and 35.8 grams of manganese nitrate were weighed, added to 57.6 grams of acrylic acid, and a homogeneous solution was formed by magnetic stirring, wherein the molar ratio of zinc sulfate, manganese nitrate and acrylic acid was 1:2:8. 0.3 ml of 5% ammonium persulfate solution was added to the above solution as an initiator, and after mixing uniformly, it was allowed to stand at 80° C. for 2 hours, so that the polymerization reaction was fully carried out, and a polyacrylate precursor was formed. The polyacrylate precursor was then dried at 120°C for 24 hours, followed by calcination at 500°C for 8 hours to obtain a brown powder product, ZnMn 2 O 4 .
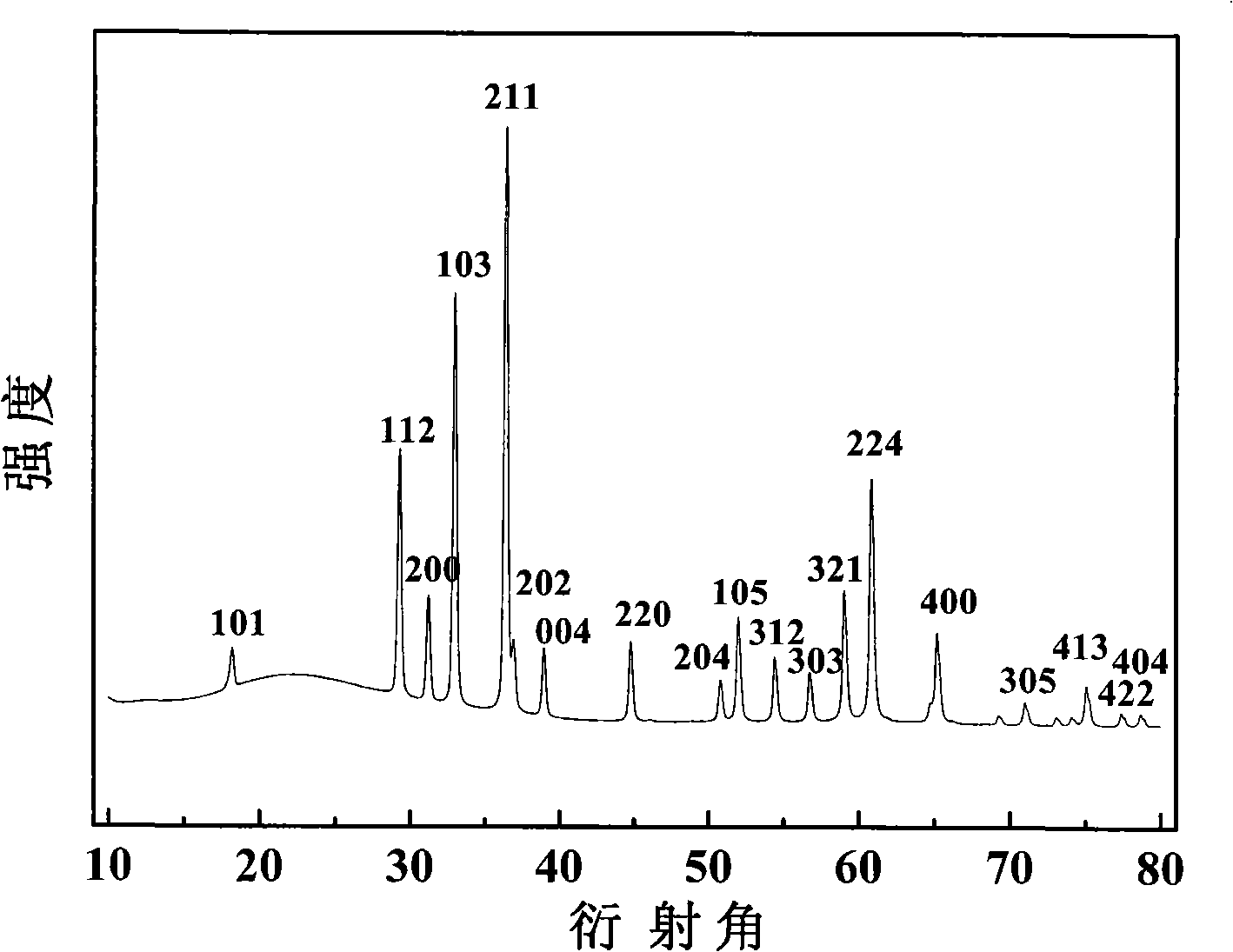
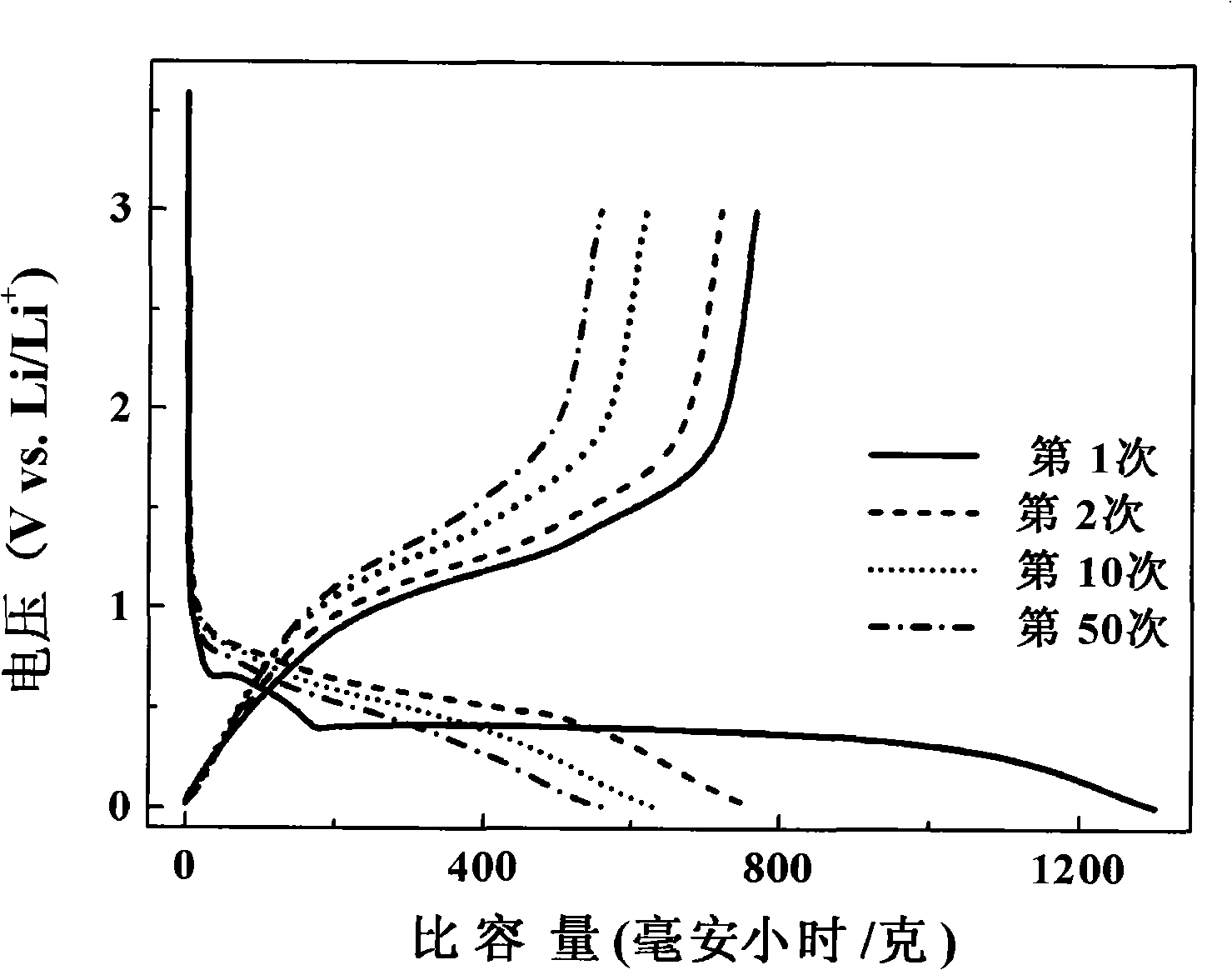
[0027] The obtained samples were observed by transmission electron microscopy (JEM-2010FEF) (see figure 1 ), the particle size of the nanoparticles is 30-60 nanometers, and the dispersibility of the material is good; the obtained sample was tested by XRD (Shimadzu XRD-6000), and the diffraction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com