Synthetic method of o-hydroxyacetophenone
A technology for the synthesis of o-hydroxyacetophenone and its synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of being unsuitable for industrial application, difficult to handle, and difficult to prepare catalysts, and achieve good industrial application prospects, easy operation, and high product yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
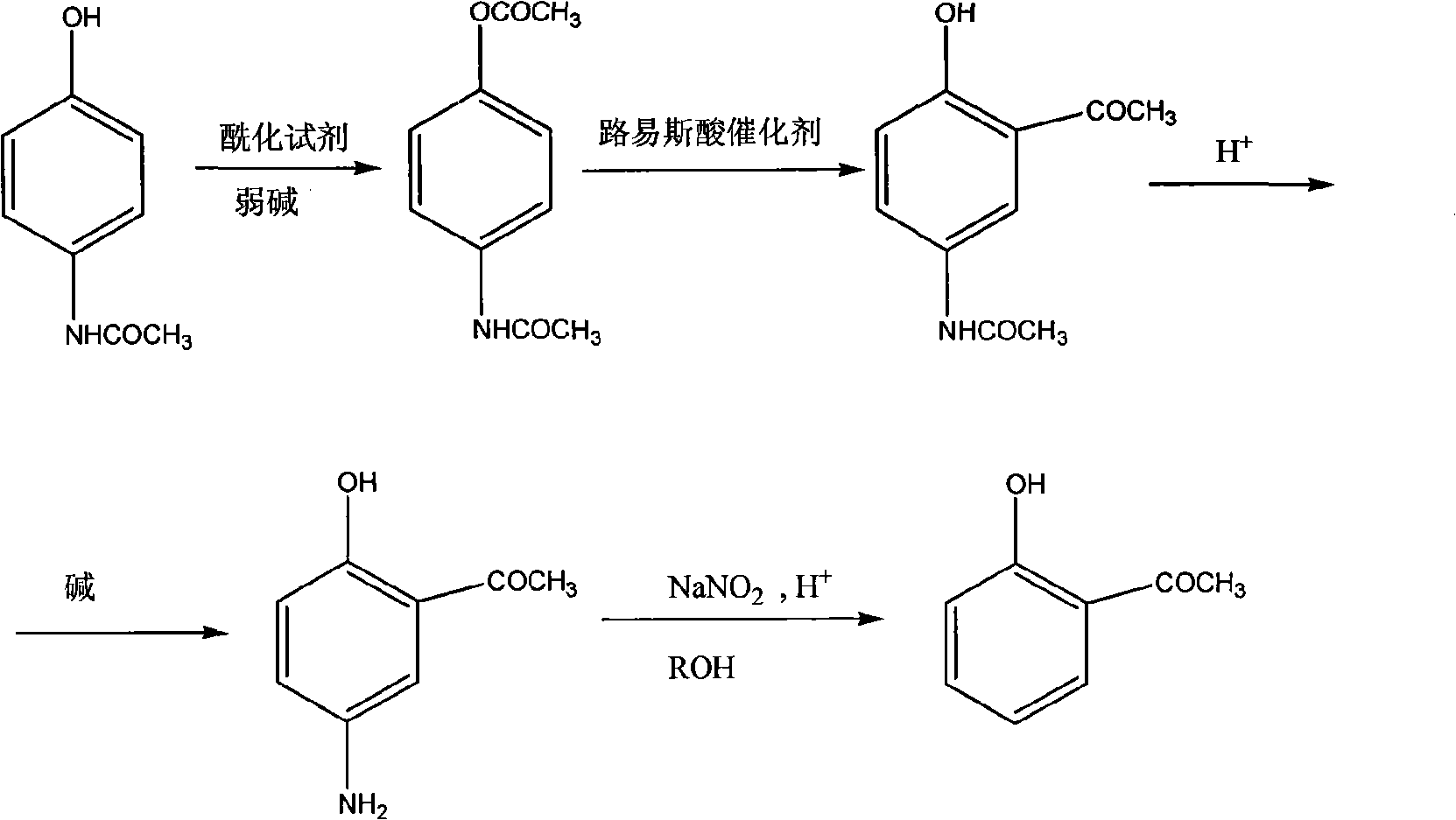
[0029] 1) Preparation of 4-acetamidophenol acetate
[0030] Add paracetamol (45.4g, 0.3mol), acetyl chloride (33g, 0.42mol) and anhydrous potassium carbonate (41.4g, 0.3mol) to 500ml of ethyl acetate, stir and heat, and reflux at 78°C for 18 hours . The recovered solvent was evaporated under normal pressure, cooled, and 350ml of water was added to precipitate a solid, which was filtered with suction and washed with water several times to obtain 55.7g of a white solid, with a yield of 96.1%. The experimental data are as follows:
[0031] mp 158-159°C; 1 HNMR (400MHz, CDCl 3 )δ (ppm): 7.50-7.47 (d, 2H, ArH), 7.33 (s, 1H, NHCO), 7.04-7.02 (d, 2H, ArH), 2.29 (s, 3H, CH 3 COO), 2.16(s, 3H, CH 3 CONH). IR(KBr)cm -1 : 3369, 3294, 1751, 1691, 1608, 1541, 1507, 1365, 1241
[0032] 2) Preparation of 2-hydroxyl 5-acetamidoacetophenone
[0033] Dissolve 4-acetamidophenol acetate (19.3g, 0.1mol) in o-dichlorobenzene (150ml), add anhydrous aluminum trichloride (33.3g, 0.25mol), sti...
Embodiment 2
[0047] 1) Preparation of 4-acetamidophenol acetate
[0048] According to the method of step 1) in Example 1, the charging amount of acetyl chloride was 23.6g, and 53.5g of 4-acetamidophenol acetate was obtained, and the yield was 92.3%.
[0049] 2) Preparation of 2-hydroxyl 5-acetamidoacetophenone
[0050] According to the method of step 2) in Example 1, the feeding amount of anhydrous aluminum trichloride was 13.4 g, and 15.5 g of 2-hydroxyl 5-acetamidoacetophenone was obtained after the reaction, and the yield was 80.3%.
[0051] 3) Preparation of 2-hydroxyl 5-aminoacetophenone
[0052] According to the method of step 3) in the embodiment 1, the charging amount of 15% dilute hydrochloric acid is 121.6ml, after the reaction, 13.7g of 2-hydroxyl 5-aminoacetophenone is obtained, and the yield is 90.1%.
[0053] 4) preparation of o-hydroxyacetophenone
[0054] According to the method of step 4) in Example 1, the feeding amount of concentrated sulfuric acid is 49.0g, and the r...
Embodiment 3
[0056] 1) Preparation of 4-acetamidophenol acetate
[0057] According to the method of step 1) in Example 1, the charging amount of acetyl chloride was 141.3 g, and 54.8 g of 4-acetamidophenol acetate was obtained, and the yield was 94.5%.
[0058] 2) Preparation of 2-hydroxyl 5-acetamidoacetophenone
[0059] According to the method of step 2) in Example 1, the feeding amount of anhydrous aluminum trichloride was 133.5 g, and 14.5 g of 2-hydroxyl 5-acetamidoacetophenone was obtained after the reaction, and the yield was 75.1%.
[0060] 3) Preparation of 2-hydroxyl 5-aminoacetophenone
[0061] According to the method of step 3) in Example 1, the feeding amount of 10% dilute hydrochloric acid is 91.2ml, and after the reaction, 13.8g of 2-hydroxyl 5-aminoacetophenone is obtained, and the yield is 91.4%.
[0062] 4) preparation of o-hydroxyacetophenone
[0063] According to the method of step 4) in embodiment 1, NaNO 2 The charging amount is 20.7g, and after the reaction, 36.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com