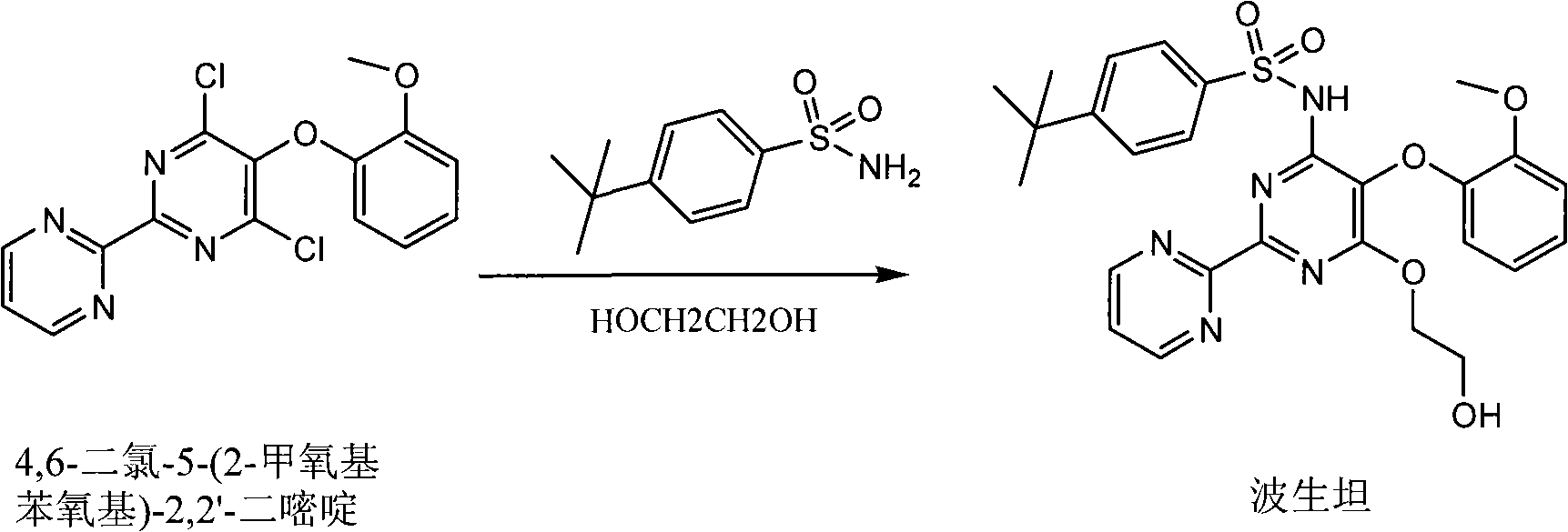
Synthetic method of 4,6- dichloro-5-(2-methoxyphenoxy)-2,2í»-dipyridine
A technology of methoxyphenoxy and dipyrimidine, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of being unsuitable for industrial production, difficult to scale up production, and low in total yield, and achieves reduction of intermediate operation steps and operation costs. , The effect of improving yield and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
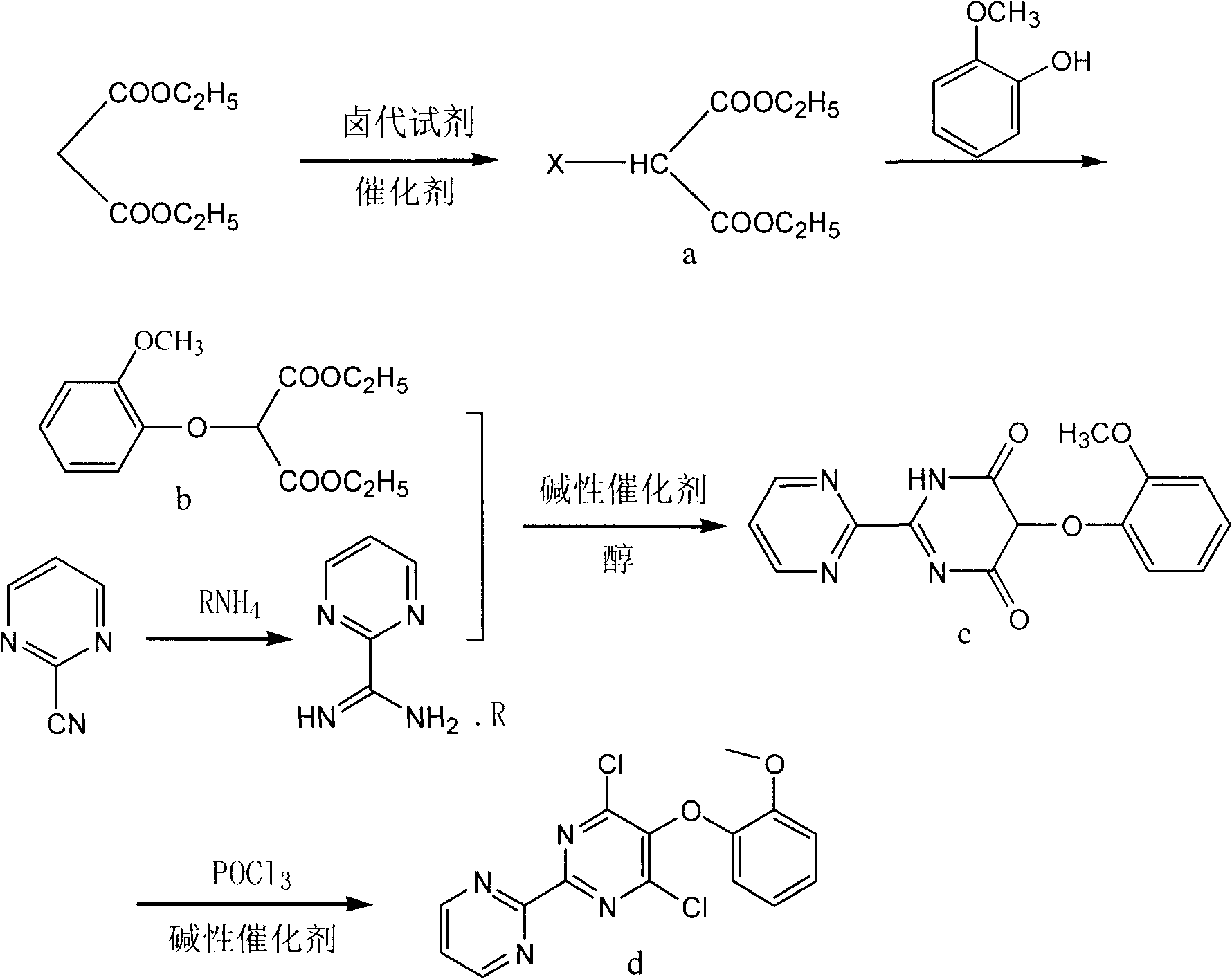
[0032] (1) Preparation of 2-halogenated diethyl malonate (a):
[0033] In a 100L reactor, add 64L of 1,2-dichloroethane, 6.4KG of diethyl malonate, 11KG of benzenesulfonic acid, and finally add 5.4KG of NCS. Raise the temperature to 60-80°C and react for 5-8 hours. Evaporate 1,2-dichloroethane under reduced pressure, then add 30L water, stir and separate the liquid, extract the water layer with 1,2-dichloroethane 20L×3, combine the organic layers, wash with 10L water, add 6KG to the organic layer Dry over anhydrous sodium sulfate, filter after 3 hours, and evaporate to dryness under reduced pressure to obtain 7.24KG of a light yellow liquid with a yield of 93%.
[0034] (2) Preparation of 2-(2-methoxyphenoxy) diethyl malonate (b):
[0035] In a 50L reactor, add 25L of acetonitrile, 3.5KG of sodium carbonate, dropwise add 2.73KG of guaiacol, and then add dropwise of 4.27KG of 2-halogenated diethyl malonate in 5L of acetonitrile solution. Control the temperature at 60-80°C an...
Embodiment 2
[0044] (1) Preparation of 2-halogenated diethyl malonate (a):
[0045] In a 100L reactor, add 64L of acetonitrile, 6.4KG of diethyl malonate, 13KG of p-toluenesulfonic acid, and finally add 7.2KG of NBS. Raise the temperature to 50-70°C and react for 4-6 hours. Acetonitrile was distilled off under reduced pressure, then added to 30L of water, stirred and separated, the water layer was extracted with 1,2-dichloroethane (20L×3), the organic layers were combined, washed with 10L of water, and the organic layer was dried by adding 6KG anhydrous sodium sulfate, 3 After one hour, it was filtered and evaporated to dryness under reduced pressure to obtain 7.75 KG of a light yellow liquid with a yield of 81%.
[0046] (2) Preparation of 2-(2-methoxyphenoxy) diethyl malonate (b):
[0047] In a 50L reaction kettle, add 25L acetone, 3.5KG sodium methoxide, dropwise add 2.73KG guaiacol, and then add dropwise 5L acetone solution of 4.27KG 2-halogenated diethyl malonate. Control the tempe...
Embodiment 3
[0056] (1) Preparation of 2-halogenated diethyl malonate (a):
[0057] In a 100L reactor, add 50L of carbon tetrachloride, 6.4KG of diethyl malonate, 8KG of methanesulfonic acid, and finally add 4.8KG of NCS. Raise the temperature to 70-80°C and react for 5-7 hours. Carbon tetrachloride was evaporated under reduced pressure, then added to 30L of water, stirred and separated, the water layer was extracted with dichloromethane 20L×3, the organic layers were combined, washed with 10L of water, the organic layer was dried by adding 6KG anhydrous sodium sulfate, and after 3 hours Filter and evaporate to dryness under reduced pressure to obtain 5.06KG of a light yellow liquid with a yield of 65%.
[0058] (2) Preparation of 2-(2-methoxyphenoxy) diethyl malonate (b):
[0059] In a 50L reaction kettle, add 40L ethanol, 2KG sodium methoxide, dropwise add guaiacol 2.7KG, then add dropwise 4.27KG 2-halogenated diethyl malonate in 5L ethanol solution, after dropping, reflux React overn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com