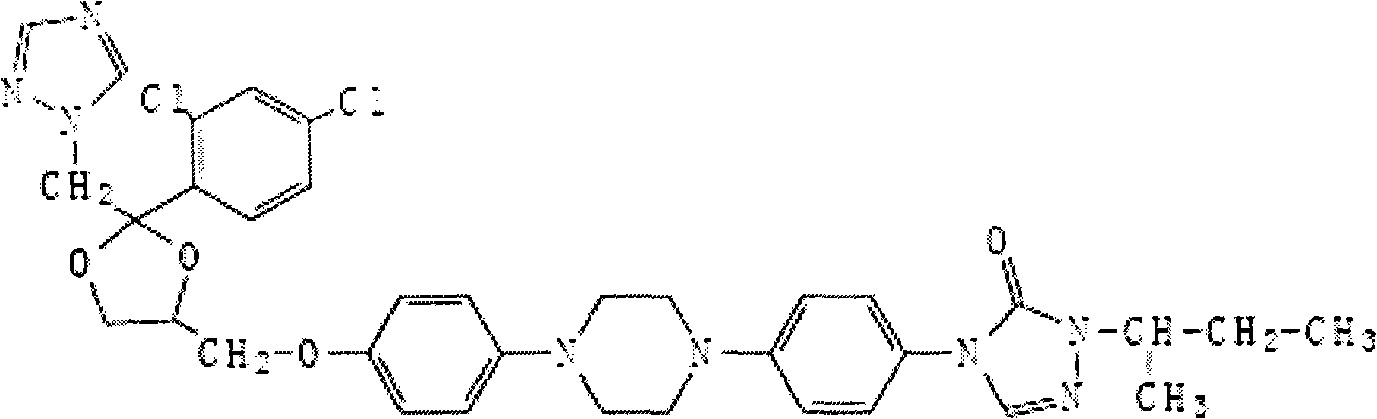
Itraconazole sustained-release drop pills and preparation method thereof
A technology of itraconazole sustained-release dripping pills and itraconazole, which is applied in the direction of medical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not being effective in time and production costs high, low dissolution rate and other problems, to achieve the effect of improving bioavailability, reducing production cost, and small difference in pellet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The preparation method of itraconazole sustained-release dropping pills of the present invention will be further described with several groups of specific examples.
[0038] First group:
[0039] Based on a total weight of 100g, weigh 30% of the matrix PEG4000, 20% of PEG6000, 20% of stearic acid, 10% of glyceryl monostearate, and 20% of the raw material itraconazole; Stir to make it melt, add the corresponding proportion of itraconazole, stir well, and under the condition of heat preservation, drop the molten or mixed liquid medicine into the condensation column filled with simethicone oil, wherein, the temperature when heating and melting The temperature of the condensate is 55°C, the temperature of the upper part of the condensate is 30°C, and the temperature of the bottom is -2°C; take it out after forming.
[0040] The obtained product has a 2-hour cumulative release percentage of 35-55%, a 6-hour cumulative release percentage of 62-82%, a 10-hour cumulative relea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com