Preparation of positive electrode material of lithium ion cell namely lithium iron phosphate
A technology for lithium ferrous phosphate and lithium-ion batteries, which is applied in electrode manufacturing, battery electrodes, chemical instruments and methods, etc. It can solve the problems of poor product reproducibility and consistency, uneven mixing, and messy particle sizes of products. Achieve the effects of easy process control, low cost, and uniform distribution of precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
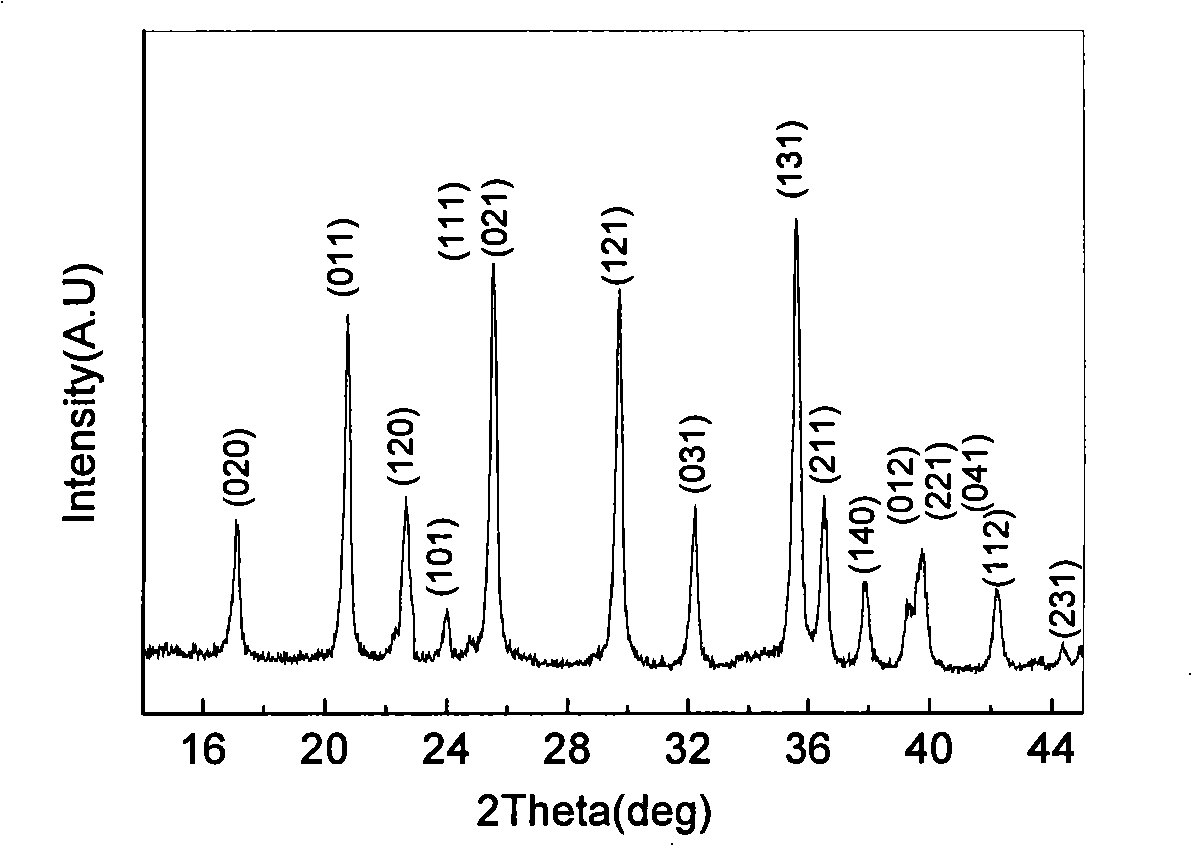
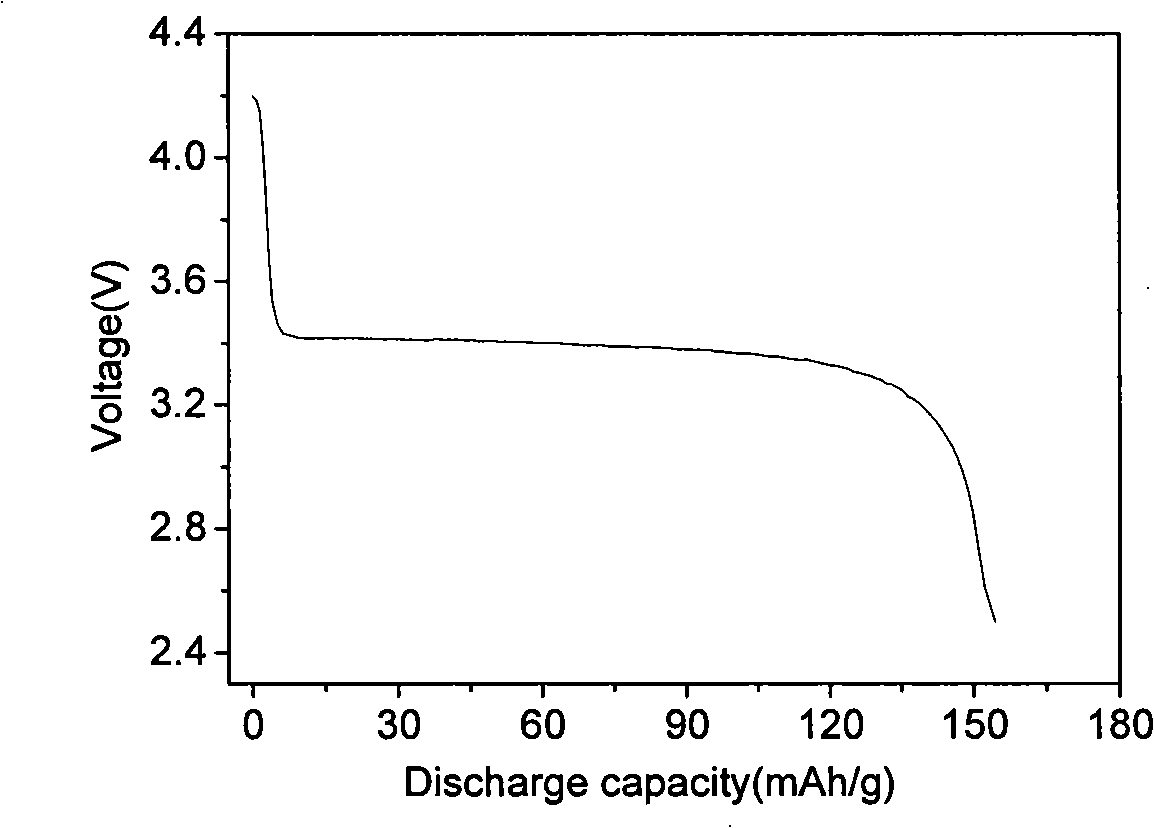
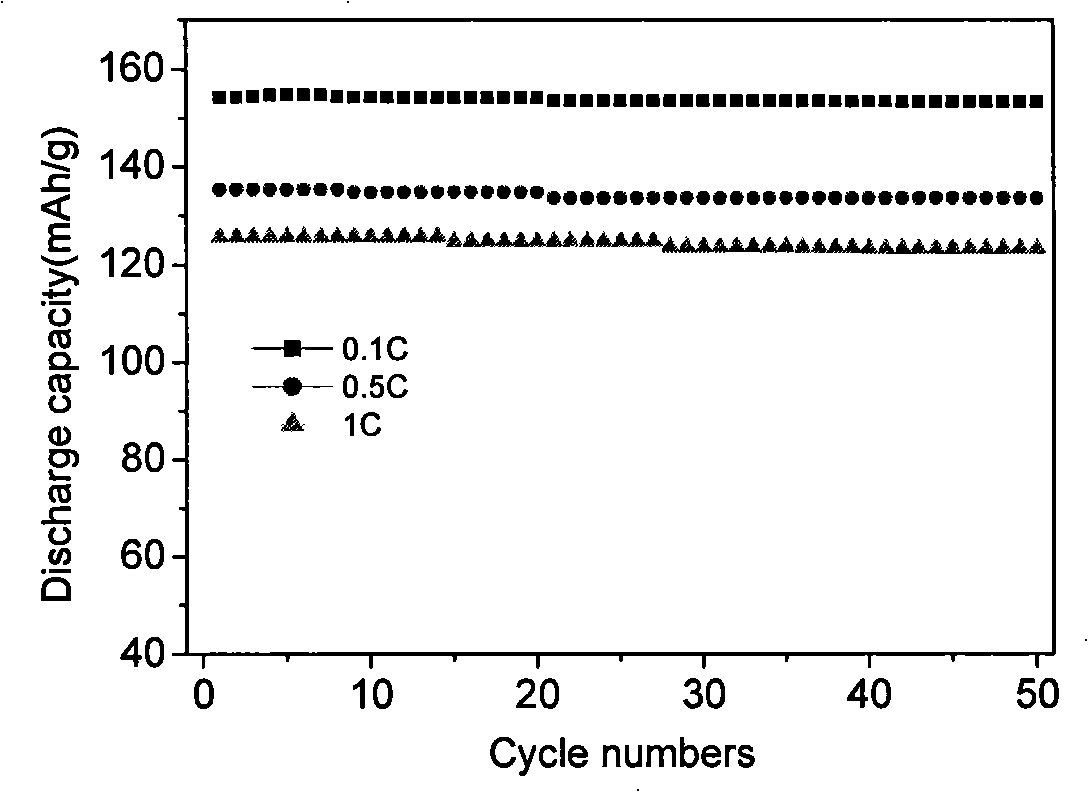
[0030] Fe 3+ Salt and H 3 PO 4 , and then add deionized water to make 0.1mol·L -1 mixed solution, and then the concentrated NH 3 ·H 2 O made into 0.3mol·L -1 solution, the ammonia solution was added to Fe under magnetic stirring 3+ and H 3 PO 4 In the mixed solution, the pH is controlled to be 2.1, and the precipitate is dried at 110°C for 3 hours to obtain FePO 4 ·xH 2 O powder. Weigh 0.1mol FePO 4 ·xH 2 O, 0.11mol lithium-containing molten salt LiOH·H 2 O and 0.061mol glucose ball milled for 5h and mixed evenly. The powder was placed in a muffle furnace at 480°C for 5 hours under an argon atmosphere, then heated to 600°C at a rate of 2°C / min, calcined for 8 hours, and cooled naturally to obtain the prepared lithium iron phosphate. Its crystal structure is shown in figure 1 . Depend on figure 1 It can be seen that the lithium iron phosphate prepared by the above method is a pure phase, and the crystallinity of the material is good.
[0031]Fully mix the prep...
Embodiment 2
[0033] Fe 3+ Salt and H 3 PO 4 , and then add deionized water to make 0.1mol·L -1 mixed solution, and then the concentrated NH 3 ·H 2 O made into 0.3mol·L -1 solution, the ammonia solution was added to Fe under magnetic stirring 3+ and H 3 PO 4 In the mixed solution, the pH is controlled at 1.9, and the precipitate is dried at 110°C for 3 hours to obtain FePO 4 ·xH 2 O powder. Weigh 0.1mol FePO 4 ·xH 2 O, 0.13mol lithium-containing mixed molten salt (LiOH·H 2 O+LiNO 3 , the former 0.1mol, the latter 0.03mol) and 0.061mol glucose ball milled for 5h and mixed evenly. The powder was placed in a muffle furnace at 300°C for 5 hours under an argon atmosphere, then heated to 600°C at a rate of 2°C / min, calcined for 8 hours, and cooled naturally to obtain the prepared lithium iron phosphate. Other tests are the same as above.
Embodiment 3
[0035] Fe 3+ Salt and H 3 PO 4 , and then add deionized water to make 0.1mol·L -1 mixed solution, and then the concentrated NH 3 ·H 2 O made into 0.3mol·L -1 solution, the ammonia solution was added to Fe under magnetic stirring 3+ and H 3 PO 4 In the mixed solution, the pH is controlled to 2.2, and the precipitate is dried at 110°C for 3 hours to obtain FePO 4 ·xH 2 O powder. Weigh 0.1mol FePO 4 ·xH 2 O, 0.13mol lithium-containing mixed molten salt (LiOH·H 2 O+LiNO 3 ,) and 0.03mol sucrose ball milled for 5h and mixed evenly. The powder was placed in a muffle furnace at 300°C for 5 hours under an argon atmosphere, then heated to 650°C at a rate of 2°C / min, calcined for 8 hours, and cooled naturally to obtain the prepared lithium iron phosphate. Other tests are the same as above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com