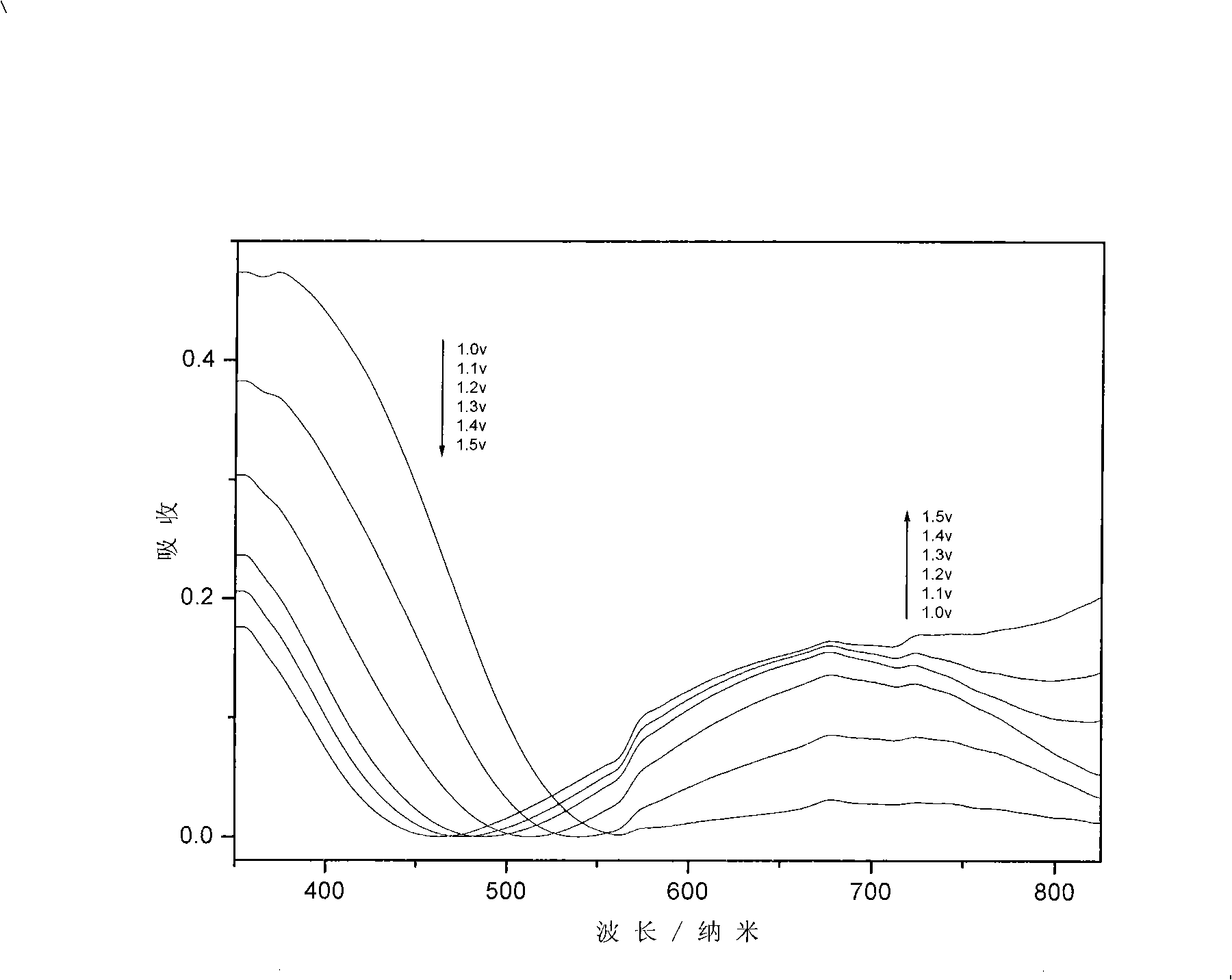
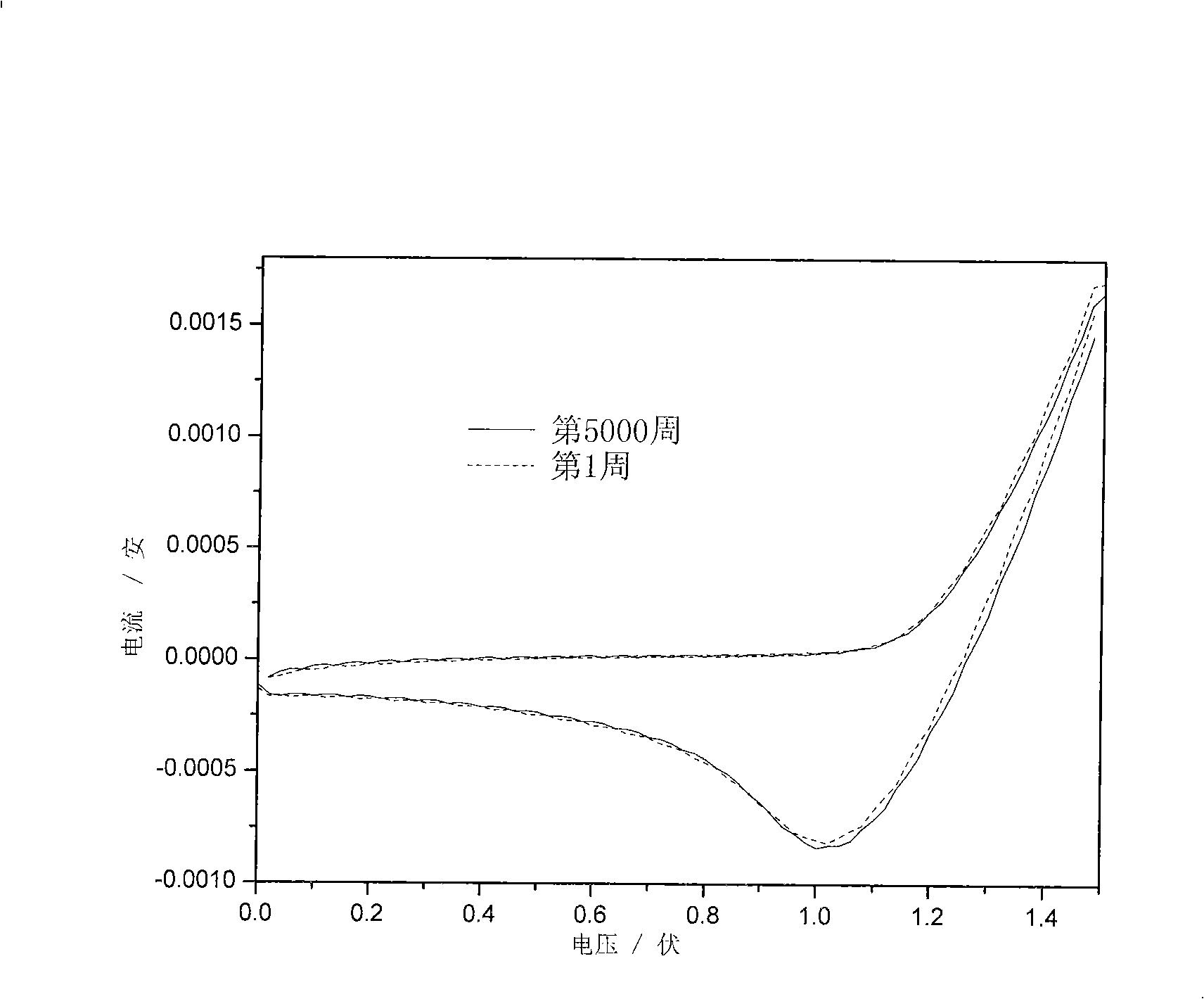
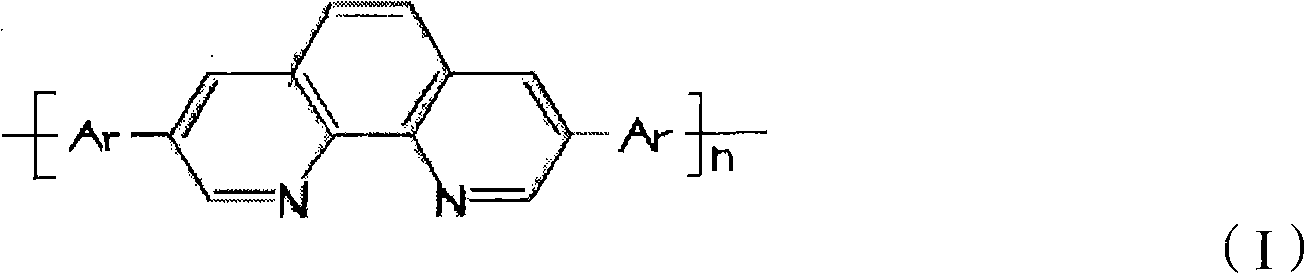Electrochromic polymeric compounds, preparing method and application thereof
An electrochromic and polymer technology, applied in the direction of electrolytic process, electrolytic components, electrolytic organic production, etc., can solve the problems of limited response time of electrochromic polymers, limited stability of electrochromic polymers, etc., and achieve response time Short and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Electrochemical synthesis of 3,8-bis(2,2'-thiophene)-o-phenanthroline polymer.
[0037] (1) Synthesis of 3,8-bis(2,2'-thiophene)-o-phenanthroline monomer
[0038] Magnesium powder (0.72 g, 30 mmol) and dry tetrahydrofuran (15 ml) were transferred to a round bottom flask under nitrogen. 2Bromo-thiophene (1.6 g, 10 mmol) was carefully added dropwise, and the mixture was allowed to react at room temperature for 2 hours under constant stirring. After filtration, the pre-prepared Grignard reagent was carefully added dropwise via cannula to the solution containing 3,8-dibromo-phenanthroline (1.34 g, 4 mmol), [NiCl 2 (dppp)] (0.070g, 0.127mmol) and dry tetrahydrofuran (15ml) in a three-neck round bottom flask and cooled to 0°C in ice water. The mixture was kept at room temperature for 2 hours and refluxed at 60°C for an additional 12 hours. with saturated NH 4 The reactant was quenched with aqueous Cl solution, extracted with chloroform, washed with sodium chloride solutio...
Embodiment 2
[0042] Electrochemical Synthesis of 3,8-Bis(2,2′-3-Methylthiophene)-Phenanthroline Polymer
[0043] (1) Synthesis of 3,8-bis(2,2'-3-methylthiophene)-o-phenanthroline monomer
[0044] Active magnesium (0.72 g, 30 mmol) and dry tetrahydrofuran (15 ml) were transferred to a round bottom flask under nitrogen. 3-Methyl-2-bromo-thiophene (1.99 g, 11.25 mmol) was carefully added dropwise, and the mixture was allowed to react at room temperature for 2 hours under constant stirring. After filtration, the pre-prepared Grignard reagent was carefully added dropwise via cannula to the solution containing 3,8-dibromo-o-phenanthroline (1.51 g, 4.5 mmol), [NiCl (dppp)] (0.070 g, 0.127 mmol) and dried tetrahydrofuran (15ml) in a three-necked round-bottomed flask and cooled to 0°C in ice water. The mixture was kept at room temperature for 2 hours and refluxed at 60°C for an additional 12 hours. with saturated NH 4 The reactant was quenched with aqueous Cl solution, extracted with chloroform...
Embodiment 3
[0048] Chemical Oxidative Synthesis of 3,8-Bis(2,2′-3-Methylthiophene)-Phenanthroline Polymer
[0049] (1) Synthesis of 3,8-bis(2,2'-3-methylthiophene)-o-phenanthroline monomer
[0050] Active magnesium (0.72 g, 30 mmol) and dry tetrahydrofuran (15 ml) were transferred to a round bottom flask under nitrogen. 3-Methyl-2-bromo-thiophene (1.99 g, 11.25 mmol) was carefully added dropwise, and the mixture was allowed to react at room temperature for 2 hours under constant stirring. After filtration, the pre-prepared Grignard reagent was carefully added dropwise via cannula to the solution containing 3,8-dibromo-o-phenanthroline (1.51 g, 4.5 mmol), [NiCl (dppp)] (0.070 g, 0.127 mmol) and dried tetrahydrofuran (15ml) in a three-necked round-bottomed flask and cooled to 0°C in ice water. The mixture was kept at room temperature for 2 hours and refluxed at 60°C for an additional 12 hours. with saturated NH 4 The reactant was quenched with aqueous Cl solution, extracted with chlorof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com