Secretory IgA and IgG antibodies-inducing agent
A secretory, inducing agent technology, applied in antiviral agents, antibody medical components, allergic diseases, etc., can solve the problems such as the inability of vaccines to obtain mucosal immune responses and insufficient antibody responses, and achieve the defense against flavivirus infection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
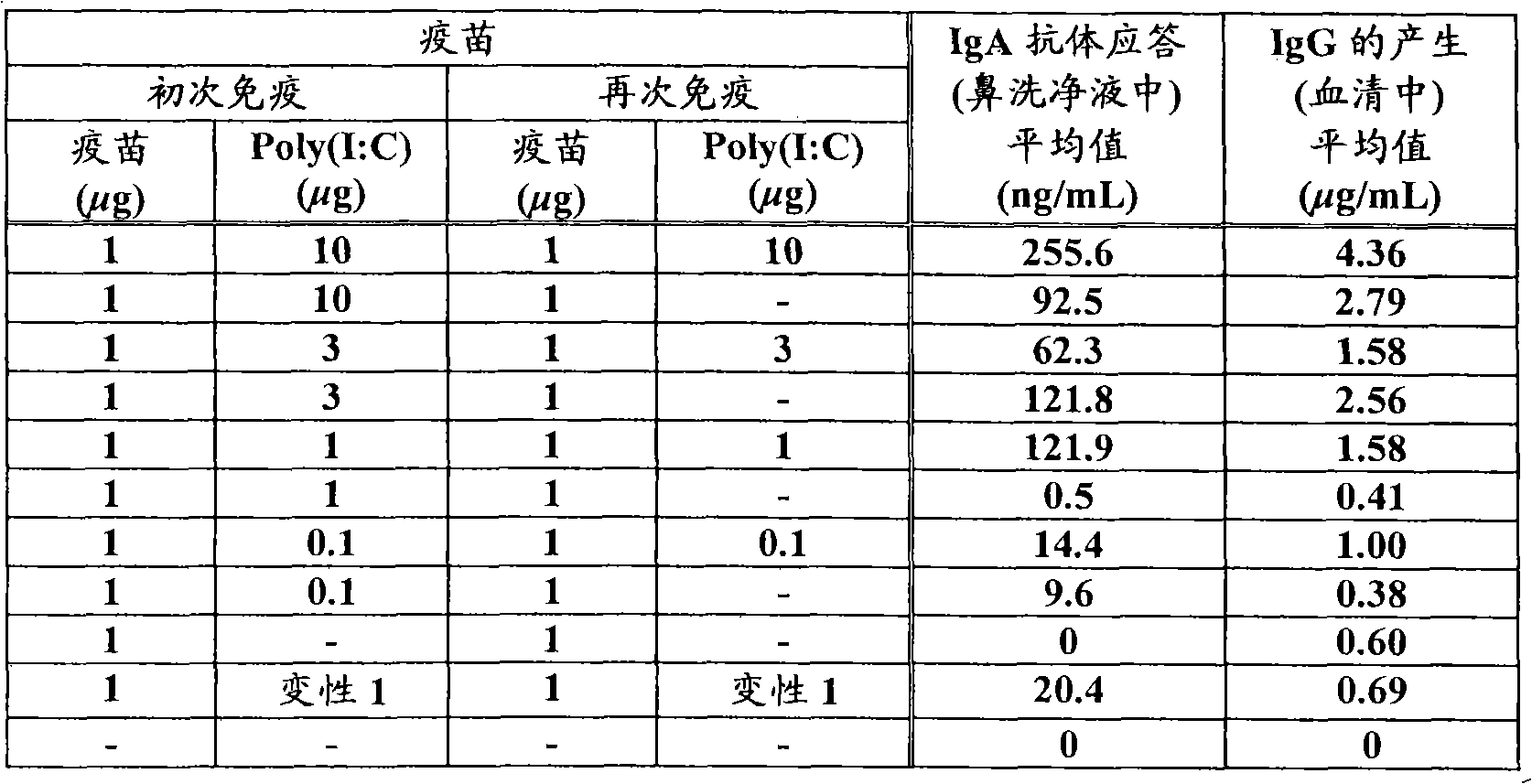
[0045] Example 1 : IgA and IgG production ability of synthetic double-stranded RNA Poly(I:C)
[0046] Synthetic double-stranded RNA Poly(I:C) was used as an adjuvant to confirm the ability of inactivated virus or subunit antigens as inactivated antigens to induce neutralizing antibodies, and then confirm the anti-pathogen effect.
[0047] (Material)
[0048] Mice: BALB / c mice (6 weeks old, female)
[0049] Virus: Hantavirus strain (obtained from National Institute of Infectious Diseases (Tokyo)).
[0050] Vaccine: Hantavirus strain (National Institute of Infectious Diseases); ether-treated inactivated vaccine
[0051] Adjuvant: CTB as positive control * [CTB (cholera toxin subunit B), containing 0.1% CT (cholera toxin)] and poly(I:C)
[0052] (method)
[0053] Five 6-week-old BALB / c mice (Nippon SLC Co., Ltd., Tokyo) were used per group. Inoculate 5 μL solution containing 1 μg hantavirus strain vaccine and 0.1 μg, 1 μg, 3 μg or 10 μg poly(I:C) adjuvant in the nasal c...
Embodiment 2
[0084] Example 2 : Efficacy of a nasal hantavirus vaccine combining inactivated virions and poly(I:C) defensive effect
[0085] (Material)
[0086] Vaccines: ether treated hantavirus HA vaccine; formalin inactivated whole virion NC vaccine
[0087] Mice: BALB / c mice (6 weeks old, female)
[0088] (method)
[0089] 0.1 μg formalin inactivated whole particle NC hantavirus vaccine combined with 0.1 μg poly(I:C) [100-1000bp: Toray] as a vaccine component of nasal hantavirus vaccine against BALB / c mice (6-week-old, female) was administered, and the same vaccine was administered again 3 weeks later.
[0090] After an additional week, the antibody responses in nasal washes and serum of the mice were measured as indicators of mucosal and systemic protective immunity, respectively.
[0091] (result)
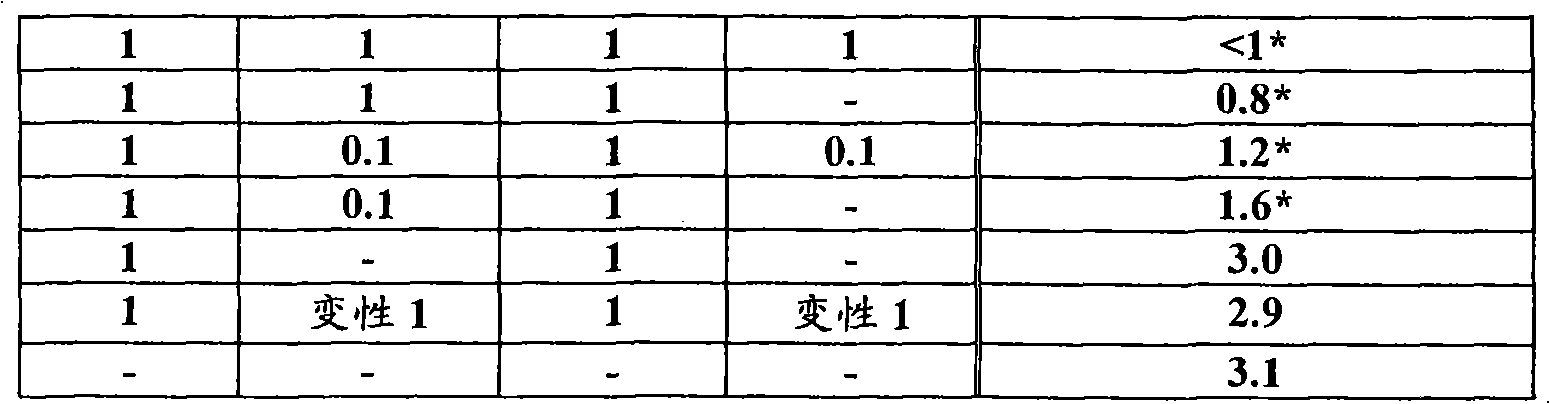
[0092] The results are shown in Table 4.
[0093] [Table 4]
[0094]
[0095] From the results in the table, it is clear that the combination of inactivated whole virions ...
Embodiment 3
[0099] Example 3 : Confirm the molecular size of poly(I:C)
[0100] (Material)
[0101] Virus: Hantavirus
[0102] Poly(I:C):
[0103] Size (L): 1~300bp (Fluka)
[0104] Size (M): 100 ~ 1000bp (Toray)
[0105] Size (H): >3.3×10 6 bp (Fluka)
[0106] Poly(A:U)
[0107] Mice: BALB / c mice (6 weeks old, female)
[0108] (method)
[0109] BALB / c mice (6 weeks old, female) were intranasally administered 0.4 μg of the lysate vaccine of hantavirus and 0.1 μg of poly(I:C) of various sizes, and the same vaccine was administered again 3 weeks later.
[0110] After another week, the antibody responses against HA and NA in nasal washes and serum of the mice were measured as indicators of mucosal and systemic protective immunity, respectively.
[0111] (result)
[0112] The results are shown in Table 7.
[0113] [table 5]
[0114]
[0115]
[0116] From the results shown in the table, it was found that the mucosal response was lower in the experimental group using poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com