Co-Th-B amorphous alloy catalyst and preparation and use thereof
A technology of amorphous alloy and catalyst, which is applied in the field of Co-B amorphous alloy catalyst and its preparation, can solve the problems of insufficient selectivity, high production cost, poor catalytic activity, etc., and achieve simple operation process and no production Effects of environmental pollution and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The pH value is greater than 13, the concentration of KBH is 2.0mol / L 4 Mixture of aqueous solution and 0.2mol / L NaOH aqueous solution KBH 4 -NaOH aqueous solution, add CoCl dropwise 2 And Th(NO 3 ) 4 In the mixed solution, where: Co 2+ With Th 4+ The mass ratio is 19:1, KBH 4 With Co 2+ And Th 4+ The molar ratio is 5:1. The temperature in the reaction system is controlled at 20°C and reacted for 30 minutes to ensure that the metal ions are completely reduced; the collected Co-Th-B catalyst is first washed with deionized water to neutrality, and then Wash with absolute ethanol, and finally store in absolute ethanol.
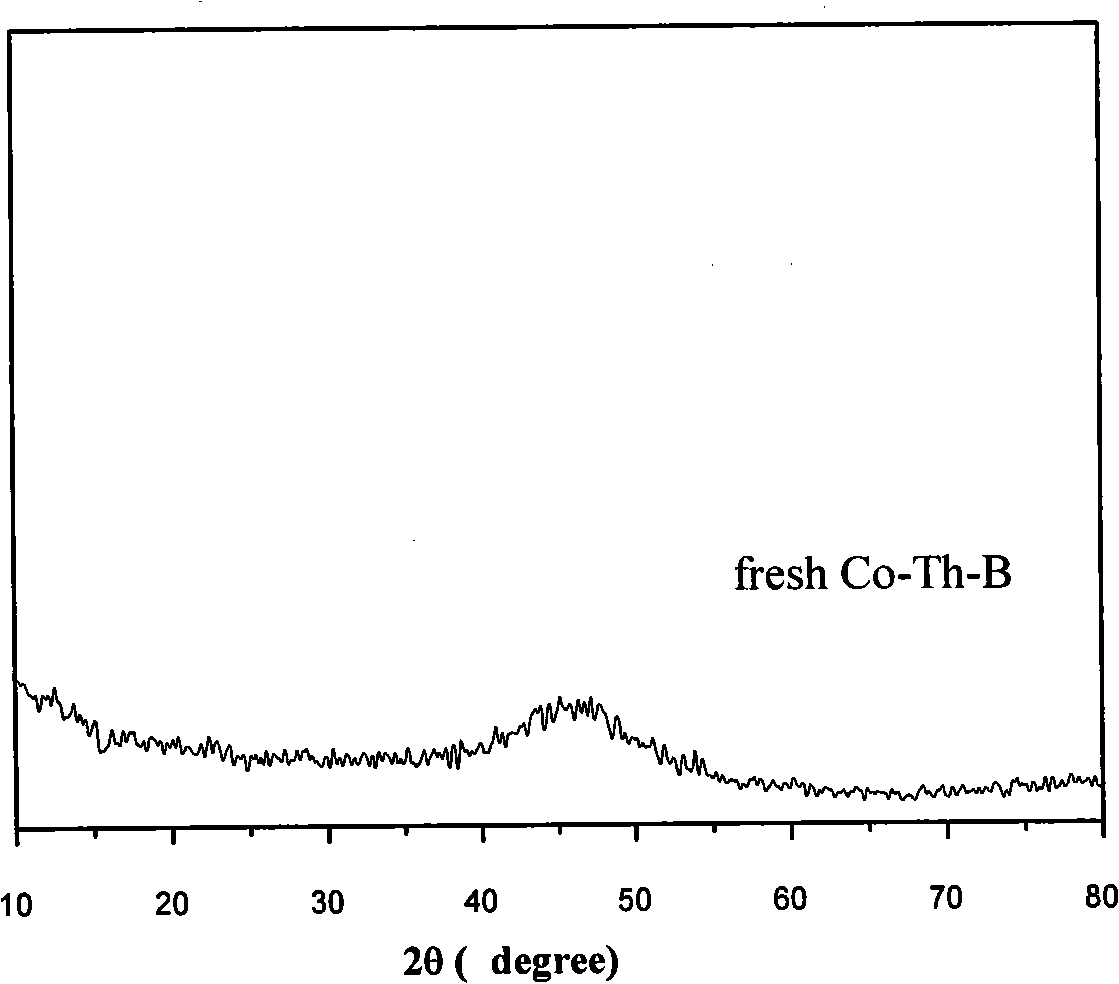
[0018] The prepared Co-Th-B catalyst is analyzed by ICP and the mole percentages of the components are: Co61.8%, Th 0.2%, B 38.0%, and its active specific surface area is 9.8m measured by the TPD method. 2 / g. The selected area electron diffraction spectrum and X-ray diffraction spectrum are shown in figure 1 with figure 2 As shown, it can be seen that the pr...
Embodiment 2
[0020] The difference between this embodiment and embodiment 1 lies in: the Co used 2+ With Th 4+ The mass ratio of is 2:1, and the rest is the same as described in Example 1.
[0021] The prepared Co-Th-B catalyst is analyzed by ICP and the mole percentages of the components are: Co57.0%, Th 1.0%, B 42.0%, and its active specific surface area measured by TPD method is 7.3m 2 / g.
Embodiment 3
[0023] The difference between this embodiment and embodiment 1 lies in: the Co used 2+ With Th 4+ The mass ratio of is 6:1, and the rest is the same as described in Example 1.
[0024] The prepared Co-Th-B catalyst is analyzed by ICP and the mole percentages of the components are: Co 59.2%, Th 0.6%, B 40.2%, and its active specific surface area measured by TPD method is 8.6m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com