Magnesium, aluminum and rare earth three-element hydrotalcite, preparation and use thereof
A technology of hydrotalcite and rare earth elements, applied in rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of poor crystallinity and difficult synthesis of products, and achieve the effect of high crystallinity and regular morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
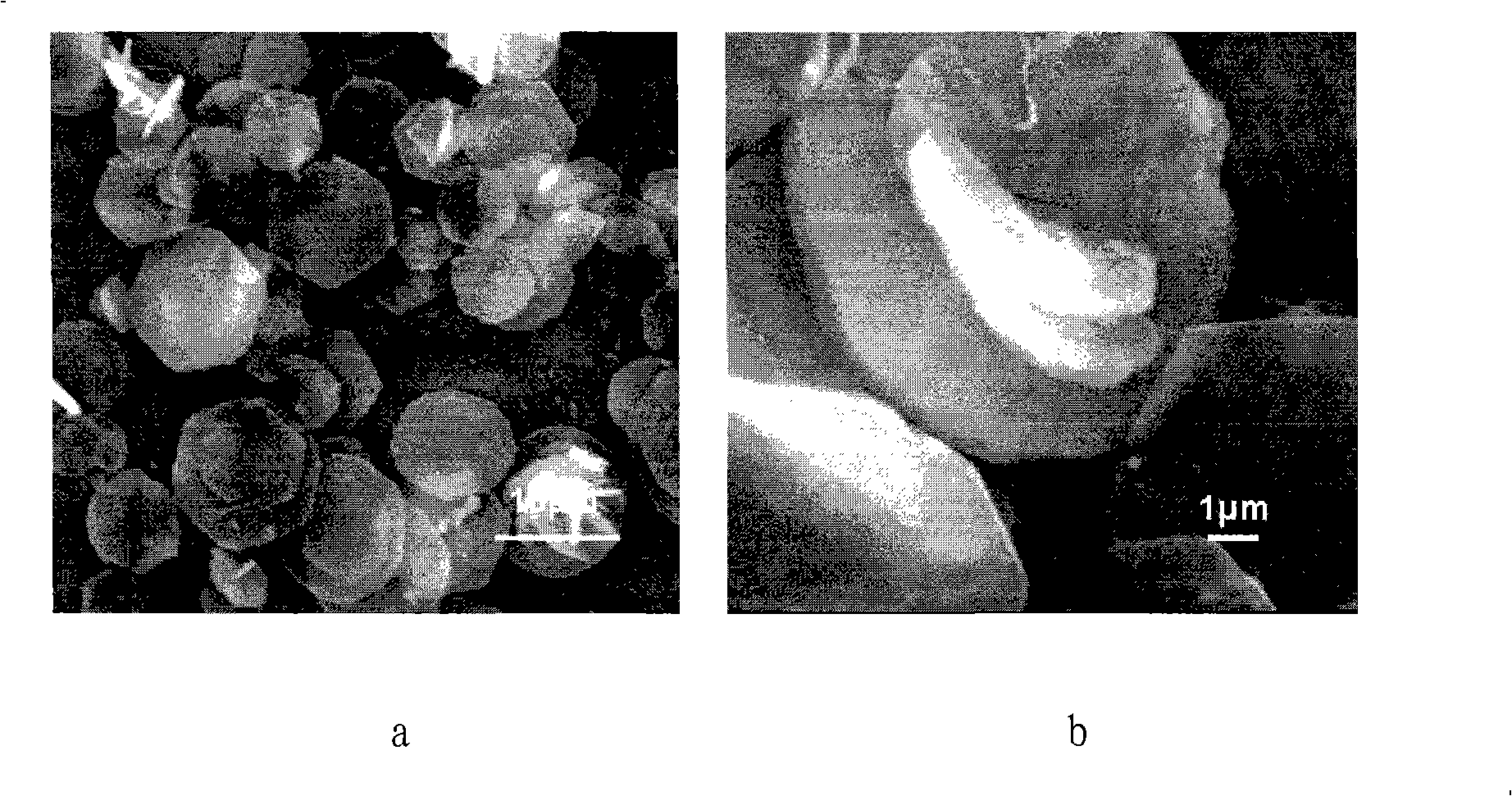
Examples
Embodiment 1
[0030] Step A: Mix 0.308g (1.2×10 -3 mol) of solid Mg(NO 3 ) 2 ·6H 2 O, 0.107g (2.85×10 -4 mol) of solid Al(NO 3 ) 3 9H 2 O, 0.008g (1.5×10 -5 mol) of solid Ce(NH 4 ) 2 (NO 3 ) 6 and 0.180g (4.0×10 -3 mol) of solid CO(NH 2 ) 2 Dissolve in 60ml deionized water. Stir the solution for 30 minutes to homogenize.
[0031] Step B: Transfer the above homogeneous solution to a hydrothermal reaction kettle with an inner substrate volume of 80ml, and tighten the lid of the kettle. The hydrothermal reaction kettle was placed in a blast drying oven at 120°C, and reacted for 24 hours.
[0032] Step C: After the reaction is complete, take out the reaction kettle and cool it to room temperature in air. The solid product was filtered, washed with deionized water, and dried in an oven at 50°C for 12 hours.
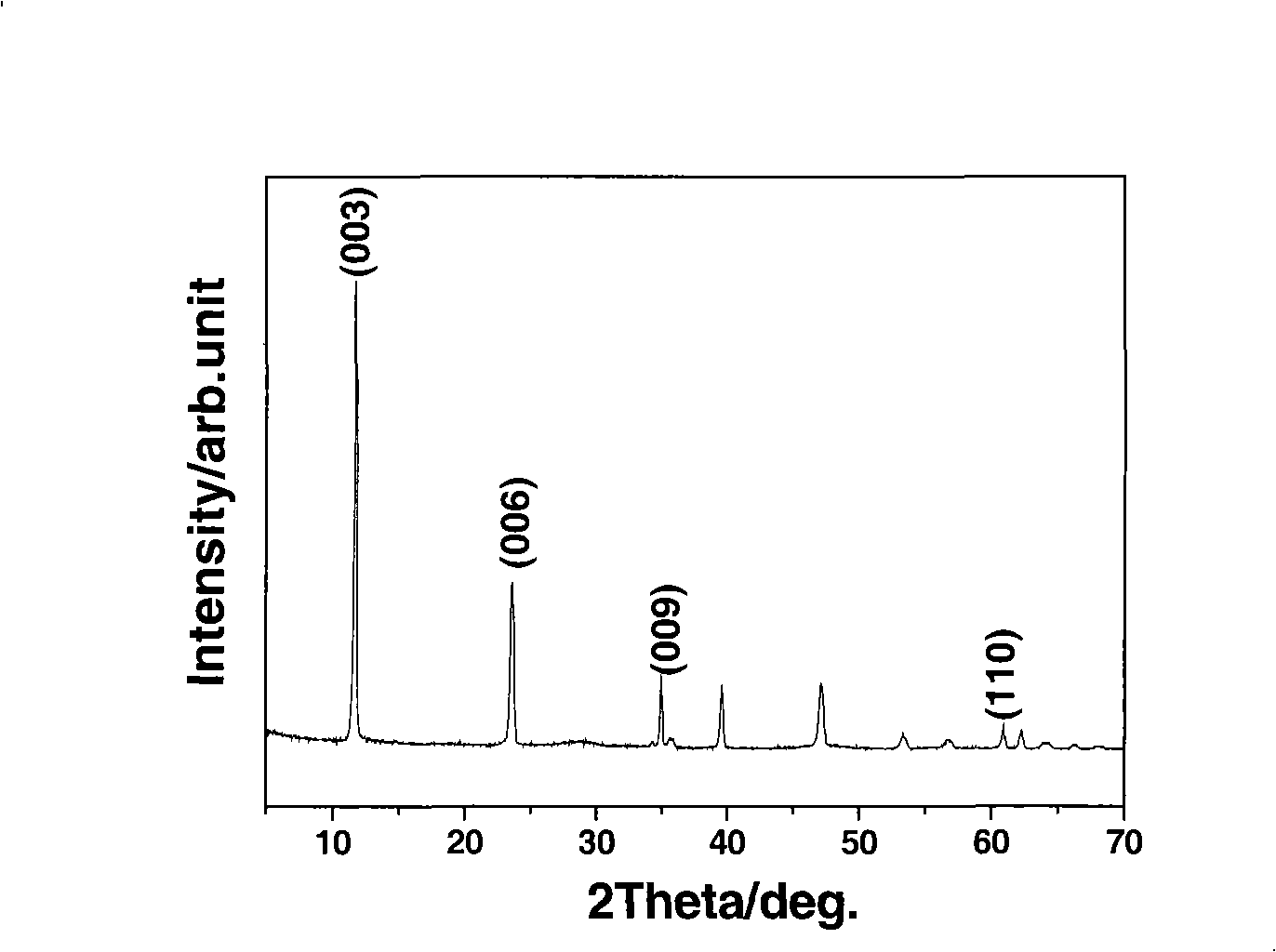
[0033] figure 1 It is the X-ray diffraction analysis (XRD) spectrogram of the magnesium aluminum cerium ternary hydrotalcite sample in embodiment 1, wherein the Mg / Al / Ce ...
Embodiment 2
[0037] Step A: Mix 0.308g (1.2×10 -3 mol) of solid Mg(NO 3 ) 2 ·6H 2 O, 0.090g (2.4×10 -4 mol) of solid Al(NO 3 ) 3 9H 2 O, 0.016g (3.0×10 -5 mol) of solid Ce(NH 4 ) 2 (NO 3 ) 6 and 0.180g (4.0×10 -3 mol) of solid CO(NH 2 ) 2 Dissolve in 60ml deionized water. Stir the solution for 30 minutes to homogenize.
[0038] Step B: Transfer the above homogeneous solution to a hydrothermal reaction kettle with an inner substrate volume of 80ml, and tighten the lid of the kettle. The hydrothermal reaction kettle was placed in a blast drying oven at 120°C, and reacted for 24 hours.
[0039] Step C: After the reaction is complete, take out the reaction kettle and cool it to room temperature in air. The solid product was filtered, washed with deionized water, and dried in an oven at 50°C for 12 hours.
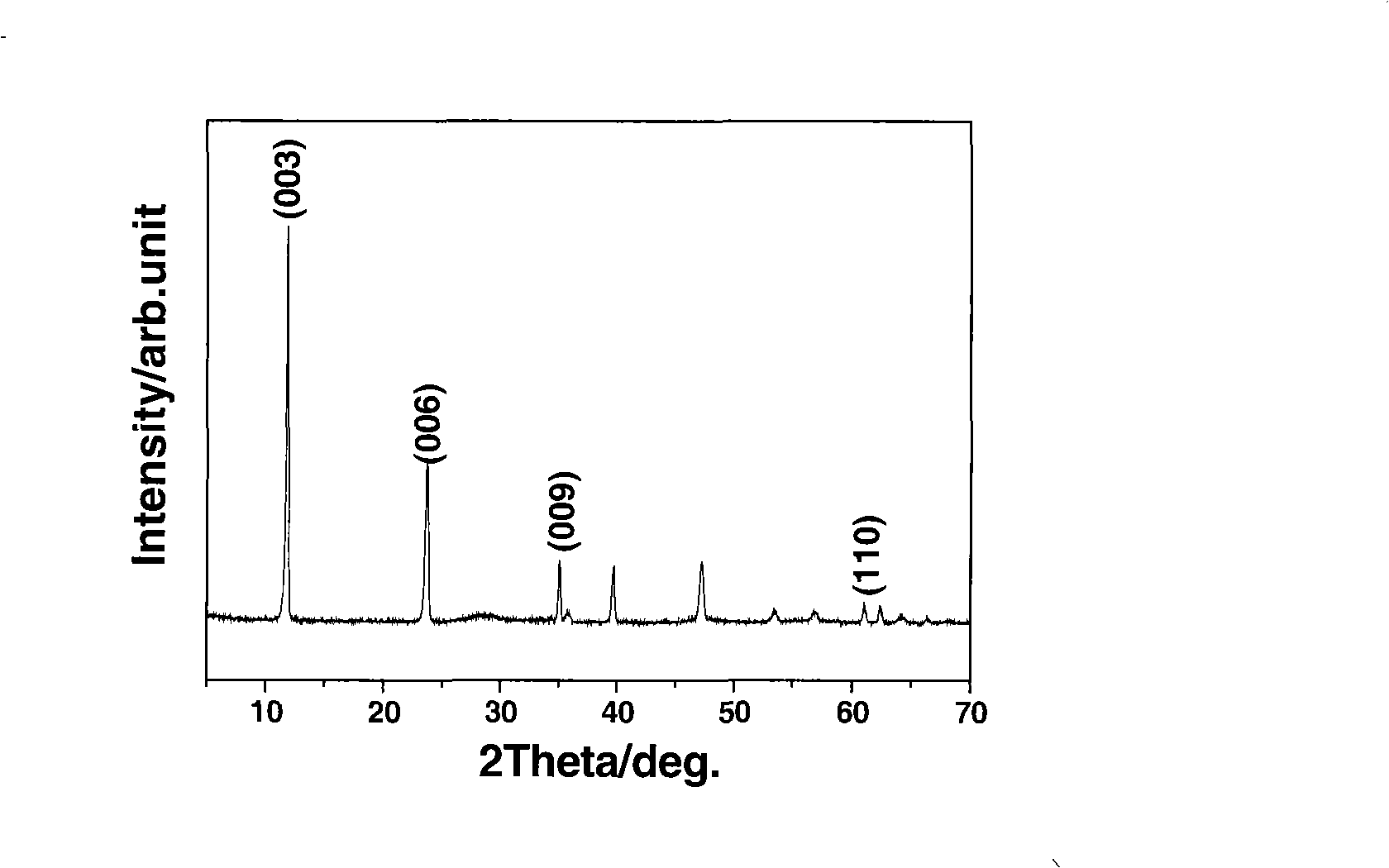
[0040] image 3 It is the X-ray diffraction analysis (XRD) spectrogram of magnesium aluminum cerium ternary hydrotalcite sample in embodiment 2, wherein the Mg / Al / Ce molar...
Embodiment 3
[0042] Step A: Mix 0.308g (1.2×10 -3 mol) of solid Mg(NO 3 ) 2 ·6H 2 O, 0.107g (2.85×10 -4 mol) of solid Al(NO 3 ) 3 9H 2 O, 0.008g (1.5×10 -5 mol) of solid Ce(NH 4 ) 2 (NO 3 ) 6 and 0.180g (4.0×10 -3 mol) of solid CO(NH 2 ) 2 Dissolve in 60ml deionized water. Stir the solution for 30 minutes to homogenize.
[0043] Step B: Transfer the above homogeneous solution to a hydrothermal reaction kettle with an inner substrate volume of 80ml, and tighten the lid of the kettle. The hydrothermal reaction kettle was placed in a blast drying oven at 120°C, and reacted for 24 hours.
[0044] Step C: After the reaction is complete, take out the reaction kettle and cool it to room temperature in air. The solid product was filtered, washed with deionized water, and dried in an oven at 50°C for 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com