Method for preparing 7-phenylacetamido-3-chloromethyl-4-carboxylic acid p-m-ethoxybenzyl ester
A technology of p-methoxybenzyl ester and phenylacetamido is applied in the field of preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester, which can solve the problems of high cost and process conditions. Harsh, complex operation and other problems, to achieve the effect of reducing investment, reducing production costs, and reducing equipment investment
Active Publication Date: 2008-11-19
JIANGSU JIUJIUJIU TECH
View PDF0 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The process conditions are harsh, the operation is complicated, and the cost is high
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
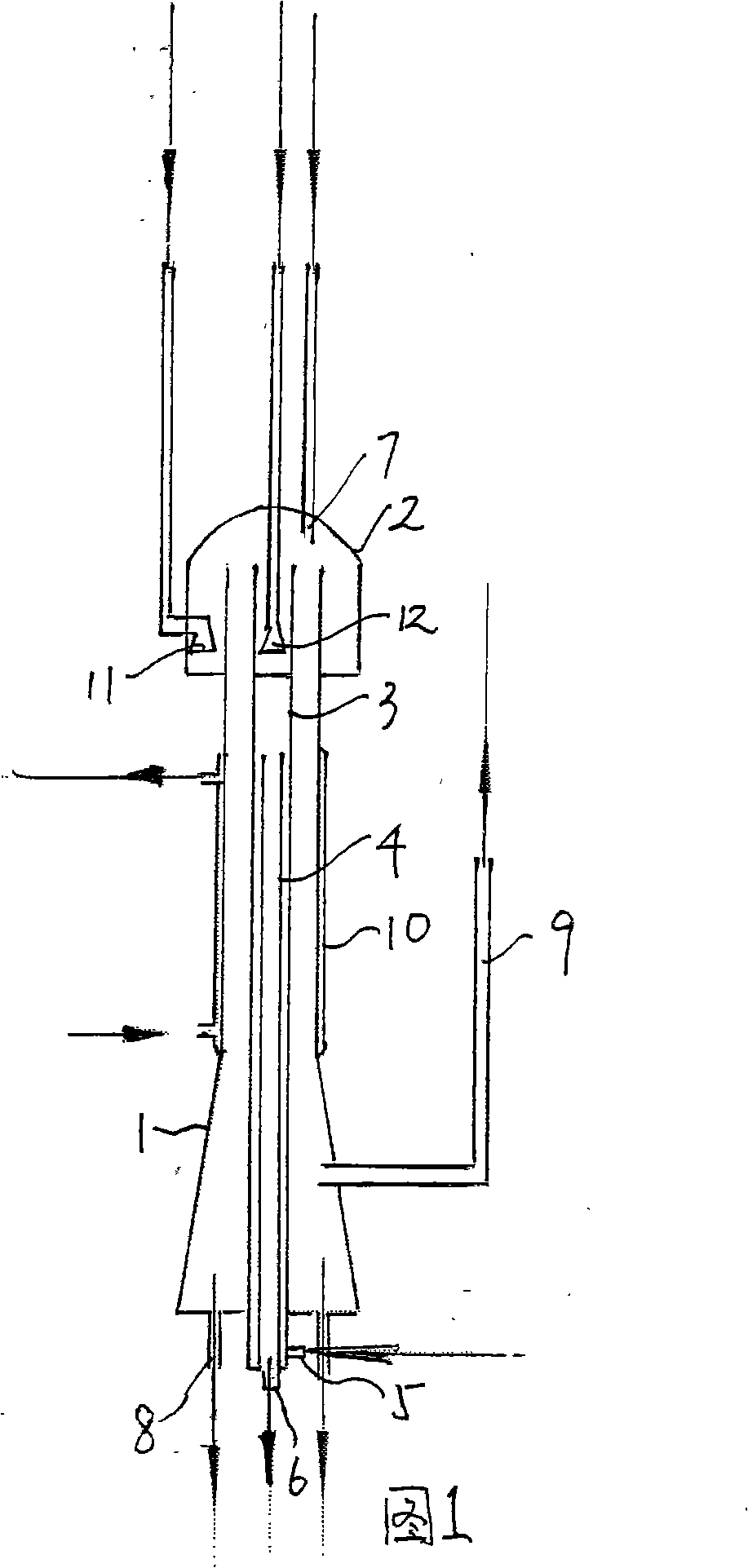
The invention discloses a method for preparing 7-phenylacetamido-3-chloromethyl-4-carboxylic acid p-meth-oxybenzyl ester. The method comprises the following steps of: adding nitrogen heterocyclic butanone sulfinic acid which is a primary standard substance, a chlorinated solvent and a catalyst which is 1 to 5 percent of the primary standard substance by weight into a reaction kettle; starting a recycle pump to cycle materials through a film chlorinator; aerating chlorine gas into the film chlorinator by using air as a carrier to perform a chlorination reaction; stopping the aeration of the chlorine gas, and withdrawing and reclaiming chlorinated solvent, when the reaction is completed; adding a ring closure solvent and dropping a ring closed agent into the film chlorinator, and adding a pH regulator into the film chlorinator at a temperature of between 20 and 25 DEG C to regulate the pH value to between 4 and 6; keeping on stirring, feeding, centrifugalizing the materials, washing the materials with solvent, and drying to produce the product. The whole process is performed at normal temperature, thereby avoiding a harsh condition of deep cooling below 40 DEG C below zero, and facilitating the industrial operation. The method reduces the investment of a deep cooling unit, reduces the equipment investment, reduces electrical load, reduces the production cost of the product, and improves market competitiveness.
Description
The method for preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester Technical field: The invention relates to a method for preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester. Background technique: In the production process of 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester, chlorination is one of the key core steps of production. At present, in production, the chlorination agent produced by electrolysis of saturated sodium chloride solution is generally used, or hypochlorite is used to carry out chlorination reaction below -40°C, and then the final product is obtained in closed loop. The process conditions are harsh, the operation is complicated, and the cost is high. Invention content: The object of the present invention is to provide a kind of process condition simple, operate respectively, the method for preparing 7-phenylacetamido-3-chloromethyl-4-cephalosporanic a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D501/24
Inventor 郑晓兵周新基朱建军赵可贵
Owner JIANGSU JIUJIUJIU TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com